Biopharmaceutical company Organicell Regenerative Medicine Inc. (OTCMKTS:BPSR) is up this morning after making an announcement regarding its product Zofin.
BPSR announced that the United States Food and Drug Administration approved the Investigational New Drug Application for the product. The trial design, which has been approved by the FDA, will start at some point in the second quarter of 2021.
Market Action
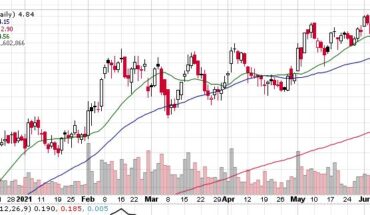
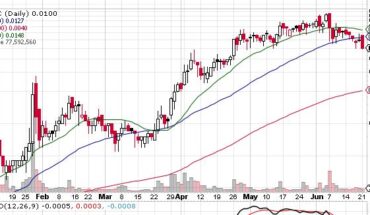
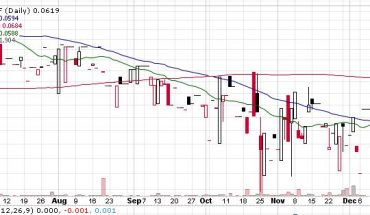
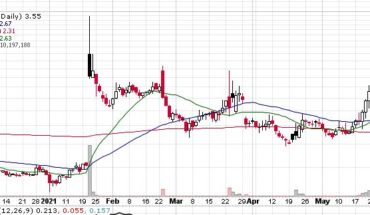
Organicell Regenerative Medicine, Inc. (OTCMKTS:BPSR) is higher in the morning session. As of 12:00, BPSR was trading at .058 up .006 for a gain of 12%. Volume is 4.4 million shares well above its 30-day average volume of 1.3 million shares. BPSR opened at .0603 and ran to a high of .085 up 50% at that time but could not hold on to most of those gains.
From the press release:- “This approved trial design, which will begin in the second quarter of this year, will be a double-blinded, placebo-controlled, phase I/II trial investigating the safety and potential efficacy of Zofin™ for patients suffering from knee osteoarthritis.”
Zofin is a product that is meant for helping patients retaining mRNAs that occur naturally. It has been made from perennial sources. At this point in time, the product is being double-blinded placebo Phase I/II trial that is going to determine its efficacy and safety. Ultimately, the product is aimed at treating patients who are suffering from COVID 19.
BPSR has been on a slide all year, down from .27 cents in December. Perhaps todays news may be what BPSR needs to turn. Put it on your watchlist to see.