InMed Pharmaceuticals Inc (NASDAQ: INM) will announce Q3 2021 revenues on May 13, 2021. It will also host a conference call at 8 AM on May 13, 2021, for the investors to discuss earnings.
A written notice to TSX for delisting
InMed sent a written message to TSX for delisting its common shares. It is on the backdrop of improving trading on NASDAQ. However, its shares will continue to get traded on the NASDAQ under the ticker INM.
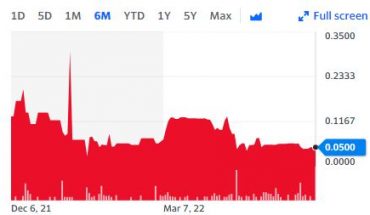
The shareholders enjoy good liquidity of INM on NASDAQ because trading volume accounts for 86% since its listing on NASDAQ on November 12, 2020.
Following the delisting on TSX with effect from May 7, 2021, the company intends to use its effort, managerial time, legal fees, and exchange fees for scientific programs and advance its business. However, the existing shareholders in Canada can trade their shares through brokers on NASDAQ.
The online and discount brokers in Canada have access to buy and sell the shares of InMed on NASDAQ.
CEO participates in 5th AGCC
CEO of InMed, Eric. A. Adams would participate in the Canaccord Genuity’s 5th AGCC (Annual Global Cannabis Conference) on May 11, 2021. Its CFO and Eric will also participate in the 1×1 meetings with family offices and institutional investor groups. The investors can reach the company by email to schedule the meeting.
Files CTA to evaluate cannabinol cream
InMed submitted CTAs (Clinical Trial Applications) in nations: Serbia, Israel, and Austria to evaluate its cannabinol cream – INM-755 cream to cure EB (Epidermolysis Bullosa). It would also submit additional CTAs for the 201 study (755-201-EB) to Ethics Committees (ECs) and NCAs (National Competent Authorities) in Italy, Greece, Germany, and France soon.
The company expects to receive responses from ECs and NCAs in August and July 2021. SVP (Clinical and
Regulatory Affairs), Alexandra Mancini, said it is thrilled to take these measures to evaluate the effectiveness of its INM-755 cream in patients with Epidermolysis Bullosa. InMed expects a positive outcome from these tests to offer benefits to the patients with EB.
The company will conduct the trial for 10 to 12 months at ten pre-qualified sites across Italy, Greece, Germany, and France by enrolling 20 patients. InMed will commence patient enrollment on receipt of the approvals and expects to receive results in H2 2022.