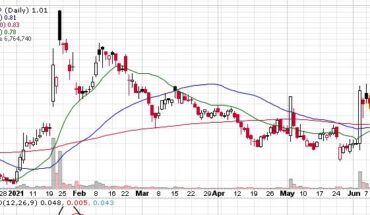
Regen BioPharma Inc (OTCMKTS: RGBP) quickly moves up on the charts with higher trading volumes of $50 million.
Best talked the small-cap stock
Around 3.5 billion shares of Regen traded on Thursday. It is one of the best talked small stocks in the market. It expects to move further higher with any break out above $0.0295. It is good news for stock traders to make smart money.
Signs a deal with Oncology Pharma Inc
KLS, a subsidiary of Regen, and Regen signed a deal with Oncology Pharma, Inc. As per the terms of the agreement, Regen granted exclusive rights and a license for the commercialization and development of a specific IP to Oncology to treat patients with pancreatic cancer. The license is valid for 15 years, with effect from April 7, 2021.
The licensed IP comprises antigen-specific cancer vaccines. Patients will receive modified mRNA to boost their immune response. mRNA produces epitopes, which are part of the antigens, which are identified by the immune system.
Focuses on immunotherapy space
Regen focuses on immunotherapy and immunology. It will advance innovative technologies by conducting Phase I/ II and preclinical studies. Regen is also working on small molecule therapies to treat autoimmune disorders and cancer through the modulation of Checkpoint NR2F6.
Regen also uses gene slicing and small molecules to cure blood disorders. It also develops new products to cure cancer using cellular immunotherapy. Other products under development at Regen will also repair bone marrow in anemia-affected patients and radiotherapy/ chemotherapy-treated humans.
Submits a patent for small molecules
Regen submitted a patent application for the small molecules, which will activate NR2F6, an innovative gene, to control immune system reactions for better inflammatory response. It also prevents cancer.
Cellular therapeutic product – HemaXellerate is developed to improve blood production in humans whose bone marrow is not correctly working. The product utilizes the own fat of the patient to treat damaged bone marrow. The US FDA cleared HemaXellerate to conduct phase I/ II clinical studies.
dCellVax stimulates the immune system of the patient through gene slicing for the treatment of breast cancer. Obtaining a safety report is essential for administering the product to patients.