CytoDyn Inc (OTCMKTS: CYDY) is moving up in the morning session, recovering from the recent sell-off.
Market Action
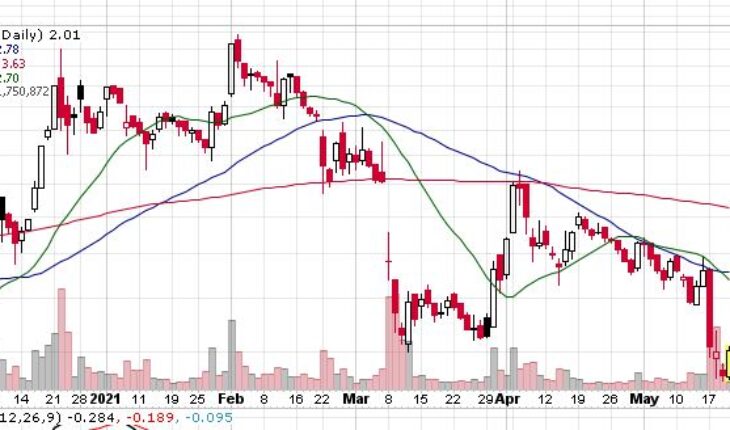
As of 11:24, CYDY stock went up 10% to $2.00. Total volume was 16.50 million shares – 4X its average volume of 4.47 million shares. The stock opened at $1.80 and moved in a range of $1.7800 – 2.0300.
CYDY Released News This Week
CytoDyn to Submit Newly Completed Topline Report of CD12 Trial Results to Regulatory Agencies in Multiple Countries including India and Philippines
- CYDY announced it intends to submit the results of its newly completed topline report of its CD12 Phase 3 clinical trial data for severe to critically ill COVID-19 patients to various regulatory agencies including but not limited to agencies in India and the Philippines.
- Patients in the CD12 trial were administered only two doses of leronlimab, the first dose at day zero and the second dose at day seven, while results were measured for 28 days (every 7 days).
- The results in the table above indicate that from day zero to day seven, critically ill patients receiving leronlimab (on day zero) experienced a mortality rate 78% lower than patients receiving placebo.
- Further, patients receiving the second dose of leronlimab achieved maximum benefit of 82% less mortality.
- However, the effects diminished from day 14 to day 21 and from day 21 to day 28, as the mortality rate decreased to 50% and 31%, respectively. This, we believe, was due to patients not being administered leronlimab past day 7.
Nader Pourhassan, Ph.D., President and Chief Executive Officer of CytoDyn, stated, “We are very thankful for the opportunity to be able to conduct two very crucial clinical trials in Brazil for severe and critically ill COVID-19 patients, which we believe could result in a statistically significant p-value of our primary endpoint leading the way to a potential approval. Although we did not meet our primary endpoint in our CD12 clinical trial in the mITT population, we were still very pleased that we did meet almost all of our secondary endpoints in the critically ill subpopulation of COVID-19 patients.