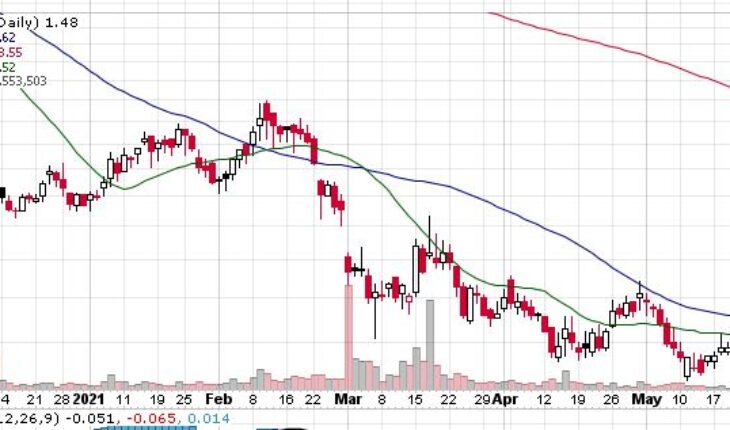
Nabriva Therapeutics plc (NASDAQ:NBRV) is one the biggest percentage gainers in pre-market trading on Wednesday.
Sinovant Sciences and Nabriva Therapeutics Announce Positive Topline Results from Phase 3 Trial of Lefamulin in Chinese Adults with Community Acquired Bacterial Pneumonia (CABP)
- announced positive topline results from Sinovant’s Phase 3 bridging study of lefamulin in Chinese adults with community acquired bacterial pneumonia (CABP).
- Sinovant’s multi-center, randomized, double-blind trial was designed to evaluate the safety and efficacy of intravenous (IV) to oral lefamulin compared to IV/oral moxifloxacin in 125 subjects with CABP. Subjects were randomized 2:1 to lefamulin and moxifloxacin and stratified by prior antibiotic exposure, pneumonia severity index (PSI) risk class, and renal impairment.
- Study drugs were dosed in double-dummy double-blinded fashion (lefamulin: 150 mg IV every 12 hours, 600 mg oral every 12 hours; moxifloxacin: 400 mg IV once daily, 400 mg oral once daily).
- Lefamulin met the primary endpoint of non-inferiority vs. moxifloxacin for Investigator Assessment of Clinical Response at Test of Cure (IACR-TOC) in the modified intent to treat (mITT) population, with success rates of 76.8% (n = 63/82) for lefamulin and 71.4% (n = 30/42) for moxifloxacin. This finding was consistent across subgroups.
- On the key secondary endpoint of IACR-TOC in the clinically evaluable (CE) population, success rates were 86.0% (n = 49/57) and 86.2% (n = 25/29) in the lefamulin and moxifloxacin arms, respectively. These results are similar to those observed in the global Phase 3 LEAP 1 and LEAP 2 clinical trials conducted by Nabriva.
Market Action
As of 7:58, USWS is trading at $2.30 up 82 cents or 55.41%. So far more than 6.13 million shares have exchanged hands.