Innovation Pharmaceuticals Inc (OTCMKTS:IPIX) is moving higher in afternoon session on Thursday after the news.
Patient Enrollment Reaches 90 Percent in Innovation Pharmaceuticals Phase 2 Clinical Trial of Brilacidin for COVID-19
- IPIX announced that patient enrollment in the Company’s 120-patient, Phase 2 clinical trial of Brilacidin for COVID-19 has reached 90 percent and that the Company anticipates full enrollment to be completed in approximately two weeks.
- Innovation Pharma is developing Brilacidin for treatment of moderate-to-severe COVID-19 in hospitalized patients under U.S. FDA Fast Track designation (see NCT04784897). The Phase 2 study is a randomized, double-blind, placebo-controlled clinical trial evaluating Brilacidin, a synthetic, non-peptidic small molecule drug candidate in a new class of compounds called defensin-mimetics, at domestic and international sites.
- The Company intends to provide an update to shareholders upon the final patients entering the trial.
About Innovation Pharmaceuticals
Innovation Pharmaceuticals Inc. (IPIX) is a clinical stage biopharmaceutical company developing a world-class portfolio of innovative therapies addressing multiple areas of unmet medical need, including inflammatory diseases, cancer, infectious diseases, and dermatologic diseases.
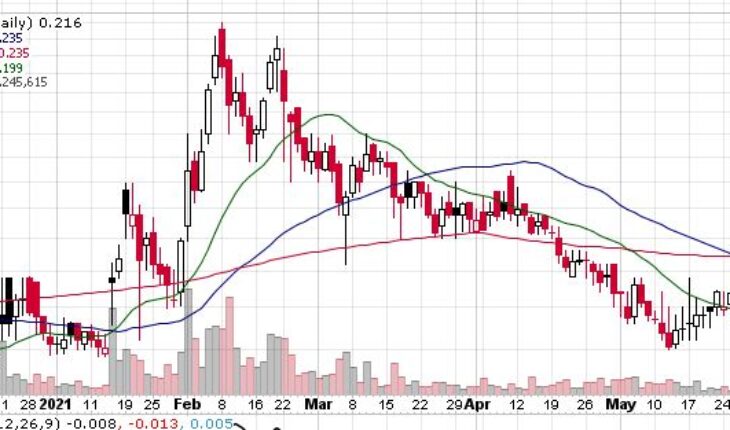
Market Action
As of 1:25, IPIX stock has moved up 3.20% to $0.2160. The stock has traded 1.23 million shares, compared to its average volume of 1.15 million shares. After opening at $0.2010, the stock has moved within a wide range of $0.1998 – 0.2190.