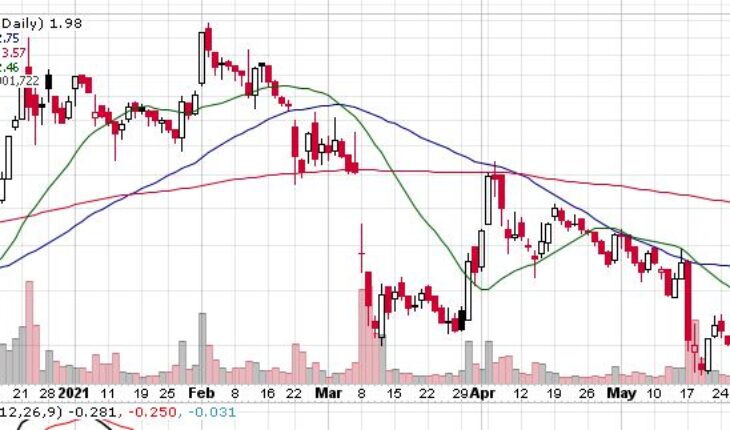
CytoDyn Inc (OTCMKTS: CYDY) continues to move lower after falling about 30% in the past couple of weeks.
Market Action
As of 1:10, CYDY stock was up down 2.50% to $1.97. The stock has witnessed a total volume of over 796K shares traded hands -compared to its average volume of 4.21 million shares. The stock opened at $2.02 and moved in a very wide range of $1.9600 – 2.0200.
Biomm S.A. Announces Plans to Submit Authorization to Conduct Phase 3 Clinical Studies of Leronlimab with ANVISA in Brazil in the Next Few Days
- CYDY’s Brazilian distribution partner, Biomm S.A. (BVMF: BIOM3), plans to submit an authorization request to the Brazilian National Health Surveillance Agency (“ANVISA”) in the next few days to conduct two Phase 3 clinical trials of leronlimab in Brazil for COVID-19.
- The two Phase 3 trials consist of trials for each severe and critically ill COVID-19 patients. Once approved by ANVISA, the Phase 3 trials will be conducted by Albert Einstein Israelite Hospital, an Academic Research Organization in Brazil.
- The COVID-19 trials in Brazil are intended to provide ANVISA with the requisite data to consider advancing the availability of leronlimab to thousands of Brazilians infected with COVID-19. These two Phase 3 trials will be conducted in up to 45 clinical sites. The critically ill protocol is for 306 patients while the severe protocol is for 594 patients, and interim analysis for both populations will be conducted when enrollment reaches about 40% of the total number for each trial.
Nader Pourhassan, Ph.D., President and Chief Executive Officer of CytoDyn, stated, “We continue to be grateful to Mr. Marchezini and his team at Biomm for expediting our efforts to advance the availability of leronlimab for all patients who might benefit from this immune modulator product. We look forward to continuing to identify partnerships in other countries experiencing a surge in COVID-19 cases similar to Brazil, the Philippines and India where leronlimab could potentially save lives. We will update investors on the next investment community call on the design of these trials based on the wealth of information we have learned from our previous COVID-19 trials.”