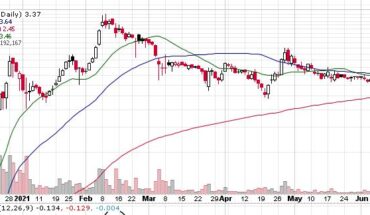
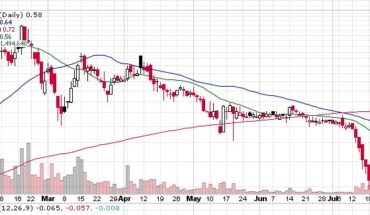
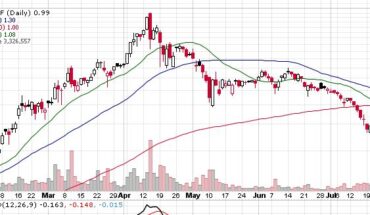
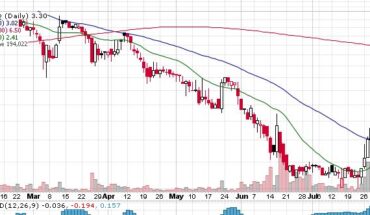
Novan, Inc. (NASDAQ: NOVN) recently announced financial results for Q1-2021 for the three months ending March 31, 2021. The clinical development stage biotech company also reported its operating results and gave a corporate update to all stakeholders. The net loss of the company for Q1-2021 is reported at $9.0 million, compared to a net loss of $6.2 million in Q1-2020. Novan’s cash and cash equivalent balance for the quarter ending March 31, 2021, is $32.7 million, while its working capital for the period is $32.6 million.
The company reported $0.7 million of collaboration and license revenue for Q1-2021 as against $1.0 million for Q1-2020. Its R&D expenses came in at $6.4 million in Q1-2021 as against $4.9 million in Q1-2020. The increase in research and development expense is attributed to the ongoing B-SIMPLE4 Phase 3 trial for molluscum treatment.
Corporate updates of Novan, Inc. (NASDAQ: NOVN)
The company started working on the evaluation of NVN4100, which is a new chemical entity of Novan. The company is exploring its potential as a product candidate eligible for antimicrobial indications for animal health companions.
Additionally, the company has announced that the first patient in its crucial study for SB206 as molluscum contagiosum has competed last Week-12 visit.
Meanwhile, Novan announced that it appointed Steven D. Skolsky as its new Board member. Steven D. Skolsky serves Novan, Inc. (NASDAQ: NOVN) as a leader with more than 35 years of experience in product development, commercialization, and strategizing.
Novan now has new corporate headquarters to assist its cGMP activities, which include R&D, along with manufacturing capabilities regarding drug product and drug substance.
In the coming quarters, the company will announce the safety and efficacy results of the B-SIMPLE4 study. This is scheduled before Q2-2021 end. Novan is scheduled to file a new drug application before Q3-2022. At present, no company has filed for FDA-approved therapies for molluscum treatment.