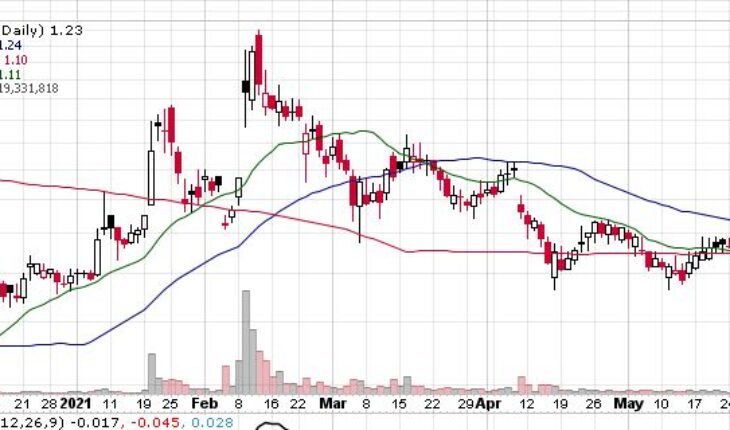
Iterum Therapeutics plc (NASDAQ:ITRM) is one of the biggest gainers in pre-market trading on Friday after the big news.
Iterum Therapeutics Provides Update on NDA Review
- ITRM announced that the Company participated in a late-cycle meeting with the U.S. Food and Drug Administration (“FDA”) yesterday.
- During the meeting, the FDA shared issues still under review regarding the Company’s new drug application (“NDA”) for sulopenem etzadroxil/probenecid for the treatment of uncomplicated urinary tract infections in patients with a quinolone non-susceptible pathogen and the Company responded to these issues. The FDA has determined that an Advisory Committee meeting is not currently necessary
- The review of the NDA is ongoing and the Company was informed that the FDA continues to work toward the PDUFA goal date of July 25, 2021.
About Iterum Therapeutics plc
Iterum Therapeutics plc is a clinical-stage pharmaceutical company dedicated to developing differentiated anti-infectives aimed at combatting the global crisis of multi-drug resistant pathogens to significantly improve the lives of people affected by serious and life-threatening diseases around the world.
Market Action
As of 7:58, ITRM is trading at $1.53, up by 34.39%. So far more than 3.11 million shares have exchanged hands.