Provention Bio (NASDAQ:PRVB) is moving lower in the pre-market session on Fridayafter the news.
Market Action
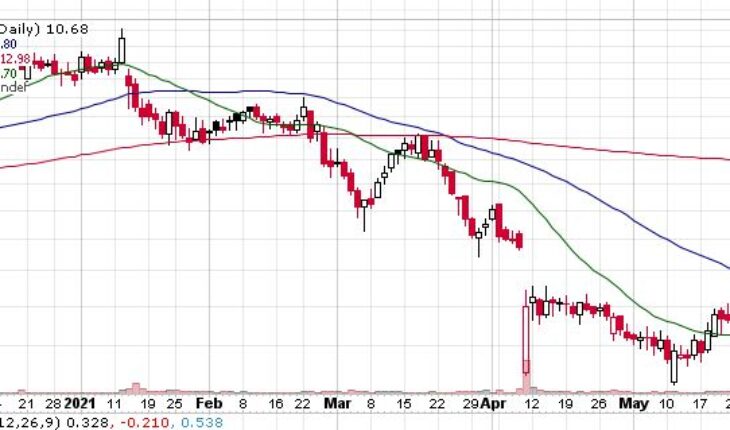
As of 7:53, PRVB stock increased 0.25% to $0.94 on low volume of 100 shares. The stock has hained almost 230% from its 52-week high of $1.20.
FDA Advisory Committee Votes in Favor of the Benefits of Teplizumab Outweighing the Risks in Support of Approval to Delay Clinical Type 1 Diabetes (T1D)
- announced that the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) of the U.S. Food and Drug Administration (FDA) voted 10 yes and 7 no on the question, “Does the information provided in the background documents and presentations by the Applicant and FDA show that the benefits of teplizumab outweigh the risks in support of approval to delay clinical type 1 diabetes mellitus?”.
- The EMDAC based its recommendation on safety and efficacy data from the pivotal TN-10 Study in which a single 14-day course of teplizumab delayed insulin-dependent, clinical-stage disease by a median of at least two years in presymptomatic patients with Stage 2 type 1 diabetes (T1D) compared to placebo. Acknowledging the significant unmet medical need facing early-stage T1D patients, the Committee Members discussed the strengths and limitations of the clinical data and provided opinions on the proposed indication statement and potential post-marketing studies.
- “We want to first and foremost thank everyone throughout the T1D community, the patients, the caregivers and the clinicians for their support today,” said Ashleigh Palmer, co-founder and CEO, Provention Bio.
- The FDA granted Breakthrough Therapy designation to teplizumab and priority review designation for the Biologics License Application (BLA). The Prescription Drug User Fee Act (PDUFA) action date is July 2, 2021. The FDA will consider today’s vote as it reviews the BLA, although it is not obligated to follow the Committee’s recommendation.