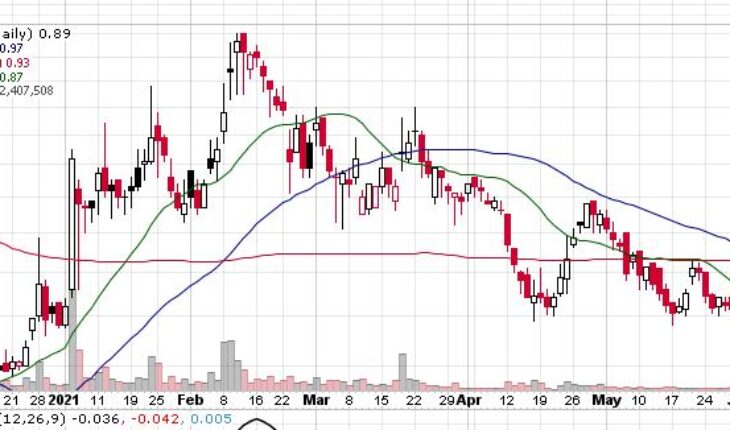
Brickell Biotech, Inc (NASDAQ:BBI) is gaining momentum Tuesday’s trading session as the stock had seen a major downtrend since the beginning of the year.
Market Action
As of 12:03, BBI stock jumped 7 cents or 9% to $0.89 with more than 2.40 million shares, well above its average volume of 1.48 million shares. The stock has moved within a range of $0.8010 – 0.9200 after opening the trade at $0.82.
Brickell Biotech Announces Publication of Japan Phase 3 Long-Term Safety and Efficacy Study Results for Sofpironium Bromide Gel, 5% (ECCLOCK®) in the Journal of Dermatology
- announced that the results from the Phase 3 long-term safety and efficacy study conducted in Japan by its development partner, Kaken Pharmaceutical Co., Ltd. (“Kaken”) were published in the peer-reviewed Journal of Dermatology1.
- The paper, entitled “A phase III, 52-week, open-label study to evaluate the safety and efficacy of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis,” is available online in English at the Wiley Online Library.
- Kaken and Brickell first announced the release of the Japan pivotal Phase 3 study results in June 2020 and this is the first release of these long-term safety and efficacy results. Kaken received regulatory approval to manufacture and market ECCLOCK® in Japan for the treatment of primary axillary hyperhidrosis in September 2020 and launched commercial sales in November 2020. Japan is the first country to approve sofpironium bromide, which also marks the first approval of a topical prescription product for the treatment of primary axillary hyperhidrosis in Japan.
- In addition, Brickell announced in its quarterly update on May 13, 2021 that it had completed enrollment in the U.S. Phase 3 Cardigan I study and exceeded 70% enrollment in the U.S. Phase 3 Cardigan II study. Both randomized, double-blinded, placebo-controlled pivotal studies are evaluating sofpironium bromide gel, 15% vs. placebo (1:1 ratio) in approximately 350 subjects (per study) aged nine and older with primary axillary hyperhidrosis.