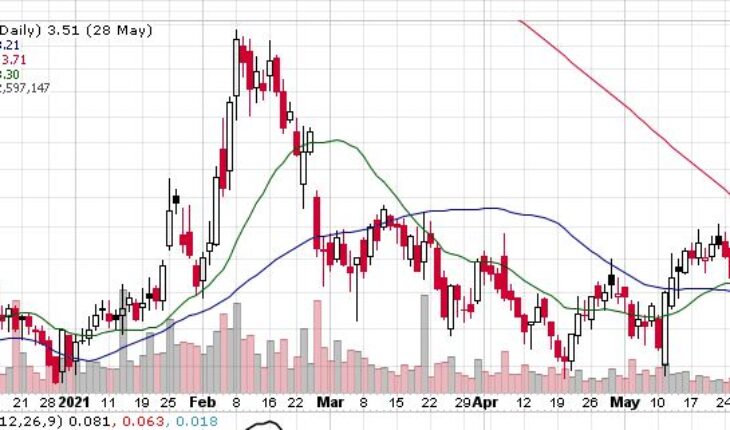
Akebia Therapeutics, Inc (NASDAQ:AKBA) is gaining momentum in the pre-market session after the news on Tuesday.
Akebia and Otsuka Announce FDA Acceptance for Filing of New Drug Application for Vadadustat for the Treatment of Anemia Due to Chronic Kidney Disease in Adult Patients on Dialysis and Not on Dialysis
- announced that the U.S. Food and Drug Administration (FDA) accepted for filing the New Drug Application (NDA) for vadadustat for the treatment of anemia due to chronic kidney disease (CKD) in both adult patients on dialysis and adult patients not on dialysis.
- The FDA has assigned the application standard review and a Prescription Drug User Fee Act (PDUFA) target action date of March 29, 2022. The FDA also indicated that they are not currently planning to hold an Advisory Committee meeting to discuss the application.
- Akebia and Otsuka are collaborating on the development and commercialization of vadadustat in the U.S., Europe, China, Russia, Canada, Australia, the Middle East, and certain other territories.
- In addition, Otsuka is working with Akebia to prepare a Marketing Authorization Application for vadadustat for submission to the European Medicines Agency expected this year.
“The acceptance of our vadadustat NDA filing marks another important milestone for Akebia and Otsuka, as we work to bring a new oral treatment option to patients living with anemia due to CKD,” said John P. Butler, Chief Executive Officer of Akebia. “We remain confident in the clarity and quality of our data, and we look forward to working with the FDA during their review of our application. In addition, we continue to collaborate with our partners to ensure we are well positioned to support a successful commercial launch of vadadustat, upon FDA approval.”
Market Action
As of 7:33, AKBA stock jumped $0.31 or 9% to $3.82. So far the stock has traded 432 shares traded hands.