CTI BioPharma Corp (OTCMKTS:CTIC) is moving higher in the pre-market session on Tuesday after the news.
Market Action
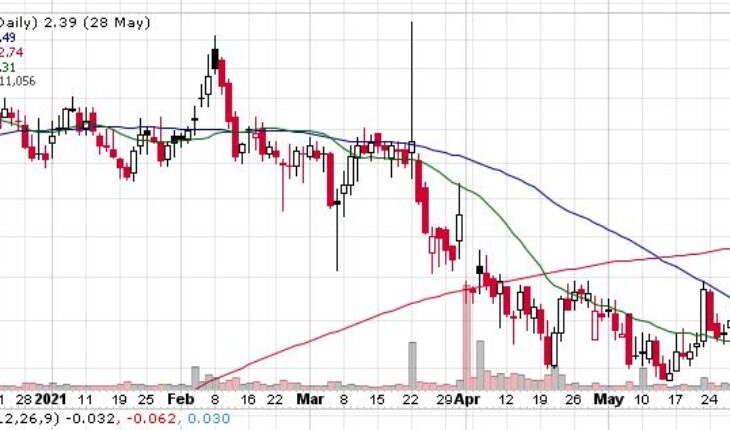
As of 7:48, CTIC stock increased 5.44% to $2.52. The stock has traded 44K shares.
CTI BioPharma Announces Acceptance of NDA Granted with Priority Review of Pacritinib for Treatment of Patients with Myelofibrosis
- announced that the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) for pacritinib as a treatment for myelofibrosis patients with severe thrombocytopenia (platelet counts less than 50 x 109/L), with the NDA being granted Priority Review. The Prescription Drug User Fee Act (PDUFA) target action date is November 30, 2021. The FDA is not currently planning to hold an advisory committee meeting to discuss the NDA.
- The NDA was accepted based on the data from the Phase 3 PERSIST-2 and PERSIST-1 and the Phase 2 PAC203 clinical trials, with a focus on the severely thrombocytopenic (platelet counts less than 50 x 109/L) patients enrolled in these studies who received pacritinib 200 mg twice a day, including both frontline treatment-naive patients and patients with prior exposure to JAK2 inhibitors.
- In the PERSIST-2 study, in patients with severe thrombocytopenia who were treated with pacritinib 200 mg twice a day, 29% of patients had a reduction in spleen volume of at least 35%, compared to 3% of patients receiving the best available therapy, which included ruxolitinib.; 23% of patients had a reduction in total symptom scores of at least 50%, compared to 13% of patients receiving the best available therapy.
- In the same population of patients treated with pacritinib, adverse events were generally low grade, manageable with supportive care, and rarely led to discontinuation. Platelet counts and hemoglobin levels were also stabilized.
“We are pleased that the FDA’s acceptance of our NDA brings us one step closer to our goal of providing myelofibrosis patients with severe thrombocytopenia a new treatment option,” said Adam R. Craig, M.D., Ph.D., President and Chief Executive Officer of CTI Biopharma. “With commercial preparation underway, we believe we will be well positioned for a potential U.S. launch later this year. We look forward to working with the FDA during its review of our application.”