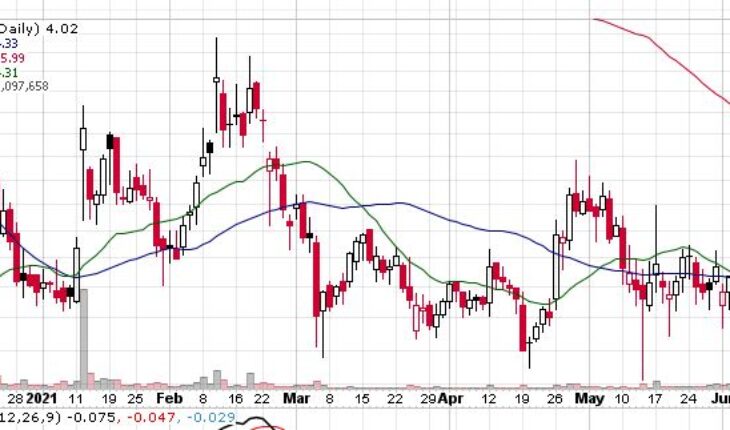
Liminal BioSciences Inc (NASDAQ:LMNL) is the biggest percentage gainer in the pre-market session after the news.
Major Trigger:
Liminal BioSciences Announces FDA Approval for its Biologics License Application for Ryplazim® (plasminogen, human-tvmh)
Key Highlights:
- announced today that the U.S. Food & Drug Administration (FDA) has approved Ryplazim® (plasminogen, human-tvmh) (“Ryplazim®”) for the treatment of patients with plasminogen deficiency type 1 (hypoplasminogenia) through its subsidiary, Prometic Biotherapeutics Inc., holder of the biological license application (“BLA”) for Ryplazim®. With this approval, Ryplazim® becomes the first FDA approved therapy for this rare genetic disorder.
- The efficacy of Ryplazim® in pediatric and adult patients with plasminogen deficiency type 1 was evaluated in a single-arm, open-label clinical trial. A total of 15 patients who had a baseline plasminogen activity level between <5% and 45% of normal were enrolled. All patients received Ryplazim® at a dose of 6.6 mg/kg administered every two to four days for 48 weeks to achieve at least an increase of individual trough plasminogen activity by an absolute 10% above baseline and to treat the clinical manifestations of the disease. Ryplazim® was well tolerated in the clinical study (See Important Safety Information).
- Efficacy was established on the basis of overall rate of clinical success at 48 weeks defined as 50% of patients with visible or other measurable non-visible lesions achieving at least 50% improvement in lesion number/size, or functionality impact from baseline. All patients with any lesion at baseline showed at least 50% improvement in the number or size of their lesions.
Market Reaction
As of 8:51, LMNL stock soared 46.27% to $5.88. So far the stock has traded 2.55 million shares traded hands.