OpGen Inc (NASDAQ:OPGN) is trading with mild gain in the pre-market session on Monday after the news.
Major Trigger:
OpGen Submits Updated 510(k) Summary to FDA and Successfully Completes Move to New Headquarters in Maryland
Key Highlights:
- announced that it has submitted an updated 510(k) summary document to the U.S. FDA for its Acuitas AMR Gene Panel for Isolates. This document incorporates all of the FDA’s requested updates to various key documents such as the Package Insert, Electronic User Guide, and Operator Manual.
- Consistent with the FDA’s previously communicated timeline, the FDA provided substantive feedback on all of these key documents by the end of May. The FDA previously informed OpGen that it intends to complete its review by the end of August 2021, but that it cannot commit to a timeline and that such timeline can be affected by various factors, including the FDA’s other workload and public health priorities.
- OpGen recently also completed the move of its U.S. headquarters, laboratories and operations as well as warehouse to its new Rockville, Maryland based facility. The Company plans to register this newly built-out facility with the FDA and other relevant authorities for the future business operations in the U.S. The new 10,000 square feet facility marks the completion of the business integration and will result in net savings of approximately $600 thousand annually in the coming years in terms of operating efficiencies and reduced rent.
Key Quote from
Oliver Schacht, CEO of OpGen commented: “We are excited to see the excellent progress that we have been able to make towards completion of our Acuitas AMR Gene Panel for Isolates filings with the FDA and the nice cadence of FDA responses and valuable inputs moving us much closer towards reaching a final FDA clearance decision point in the coming months.”
Market Reaction
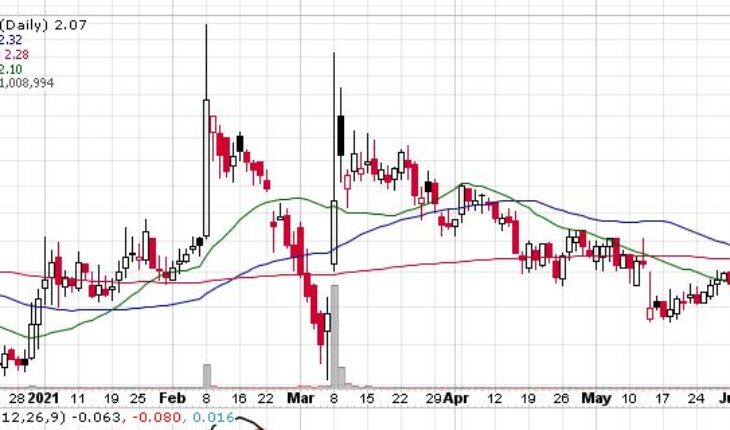
As of 8:01, OPGN is trading at $2.11, up 1.93%. So far more than 5K shares have exchanged hands.