AzurRx BioPharma Inc (NASDAQ:AZRX) is the notable gainer in the pre-market session on Monday after the news.
Market Reaction
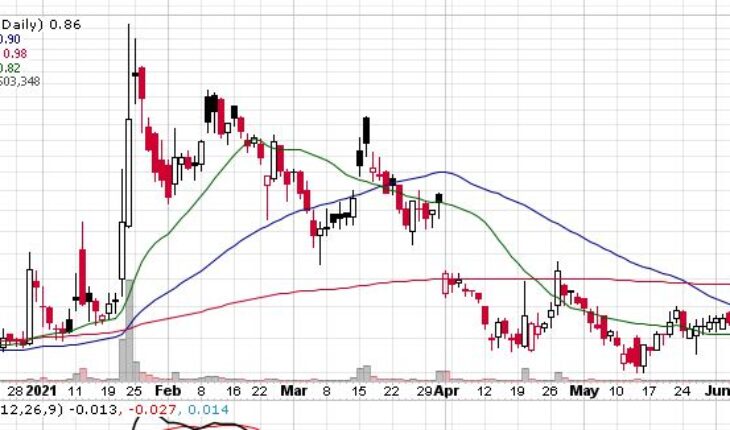
As of 7:59, AZRX stock gained 0.02% to $0.859. The stock has traded 9K shares.
Major Trigger:
AzurRx BioPharma Doses First Patient In Phase 2 Clinical Trial of Niclosamide for the Treatment of COVID-19 Gastrointestinal Infections
Key Highlights:
- announced today the administration of the first dose of FW-1022 to a volunteer in the ongoing Phase 2 RESERVOIR clinical trial. FW-1022 is a proprietary oral tablet formulation of micronized niclosamide developed for the treatment of COVID-19-related GI infections. Topline results from the trial are expected in the first quarter of 2022.
- The RESERVOIR clinical trial is designed as a two-part, two-arm, placebo-controlled Phase 2 study. The trial’s primary objectives are to confirm the safety of FW-1022 in the treatment of patients with COVID-19-related GI infections and to evaluate its efficacy in clearing SARS-CoV-2 from the GI tract.
- The primary efficacy measure of the RESERVOIR trial is the rate of fecal SARS-CoV-2 clearance (rectal swab or stool sample) assessed by RT-PCR, comparing the niclosamide arm to the placebo arm for up to six months. These long-term observation data could indicate that niclosamide treatment has the potential to improve “long haul” COVID-19 symptoms.
Key Quote from
“Dosing the first patient in the RESERVOIR clinical trial marks a significant milestone for AzurRx and the development of niclosamide as a potential treatment for COVID-19-related GI infections,” said James Sapirstein, Chief Executive Officer of AzurRx BioPharma. “There are currently no approved treatments available for COVID-19-related GI infections. If our development program is successful, we believe that FW-1022 could help prevent reinfection and the spread of COVID-19, as well as treat certain potentially severe complications that many people believe to be caused by the ability of SARS-CoV-2 to hide in reservoirs within the GI tract. We believe our micronized oral niclosamide therapy has the potential to target the virus directly in the gut and play an important role in treating COVID-19 patients experiencing the damaging aftereffects of COVID-19-related GI infection.”