Pharmather Holdings Ltd (OTCMKTS:PHRRF) stock is moving higher in the morning session on Tuesday.
Market Action
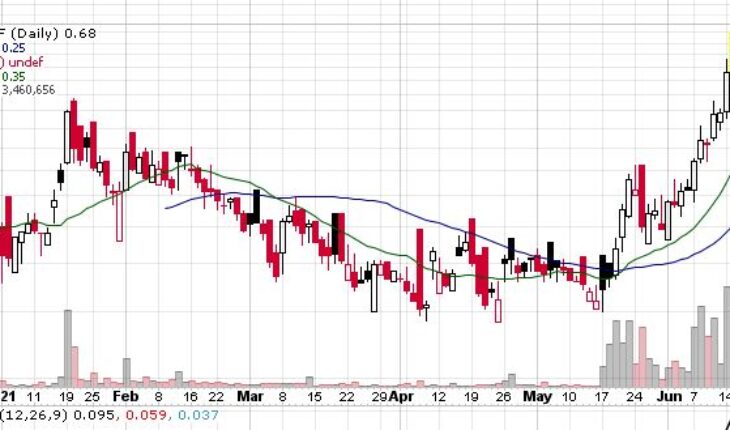
As of 10:56, PHRRF stock gained 10% to $0.6900 with more than 3.27 million shares, compared to its average volume of 630K shares. The stock has moved within a range of $0.6000 – 0.7936 after opening the trade at $0.7150.
Major Trigger:
PharmaTher Files FDA Pre-IND Meeting Request and Briefing Package For KETABET™ To Treat Depression
Key Highlights:
- PHRRF announced that it has filed a pre-Investigational New Drug (“pre-IND”) meeting request and complete pre-IND briefing package with the U.S. Food and Drug Administration (“FDA”) to support the clinical development of KETABET™ and the proposed Phase 2 clinical study as a potential next-generation treatment for depression, and to discuss the product development plan for the Company’s patented hydrogel-forming microneedle patch delivery technology.
- KETABET™ has the potential to receive FDA approval under the 505(b)(2) regulatory pathway and Fast Track designation by the FDA for treatment in patients with major depressive disorder.
- The hydrogel-forming microneedle patch offers a novel way to deliver ketamine and other psychedelics such as psilocybin, DMT, MDMA and LSD, and has the potential to improve on the safety (i.e. fewer side effects), efficacy (i.e. bioavailability, optimized dosing regimen including continuous system delivery) and compliance (i.e. storage, distribution and self-administration) of these psychedelics that currently must be taken orally, inhaled, injected and intravenously.
Key Quote:
Fabio Chianelli, Chief Executive Officer of PharmaTher, commented: “We have an insatiable focus on developing and commercializing novel uses, formulations and delivery forms of ketamine. We are committed to solving the ketamine puzzle and unlocking its true potential to treat depression by leveraging our robust intellectual property portfolio, clinical and regulatory experience, and our recently approved IND by the FDA to evaluate ketamine to treat Parkinson’s disease in a Phase 2 study.