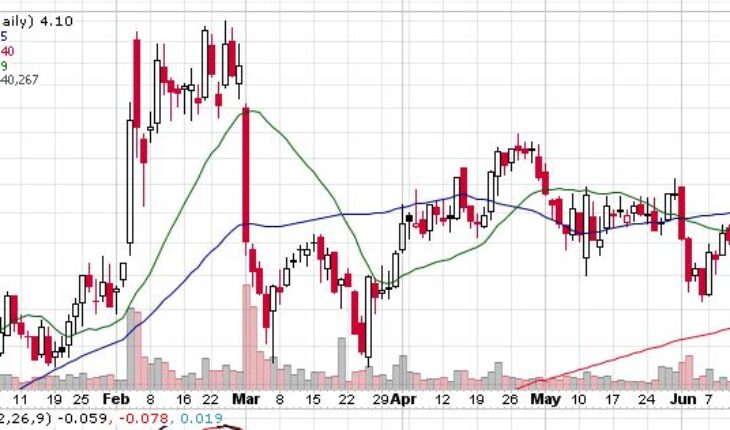
MannKind Corporation (NASDAQ:MNKD) is trading with mild gains in the pre-market session after soared gaining 5% in the past week.
Major Trigger:
MannKind and United Therapeutics Achieve a Major Milestone in the Development of Tyvaso DPI™ With New Drug Application Acceptance From the FDA
Key Highlights:
- MannKind Corporation (Nasdaq: MNKD) and United Therapeutics Corporation (Nasdaq: UTHR) reached a major milestone today with the announcement that the U.S. Food and Drug Administration (FDA) accepted for priority review of the New Drug Application (NDA) for Tyvaso DPI™ (inhaled treprostinil) for the treatment of pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD).
- The development marks the second compound formulated with MannKind’s Technosphere® technology to be reviewed by the FDA, which is expected to be complete in October 2021. The FDA has also indicated that they have not identified any potential review issues at this time.
- A next-generation formulation of treprostinil, Tyvaso DPI incorporates the dry powder formulation technology and Dreamboat® inhalation device technology used in MannKind’s Afrezza® (insulin human) Inhalation Powder, which was approved by the FDA in 2014.
Key Quote:
“We are energized by the acceptance of the Tyvaso DPI NDA for priority review,” said Michael Castagna, PharmD, Chief Executive Officer of MannKind Corporation. “MannKind is driven to deliver therapeutics in ways that can help improve patient lives for the better. With this key regulatory step, we are excited to progress the next Technosphere product towards providing thousands of PAH and PH-ILD patients a more convenient method of treprostinil therapy administration.”
Market Reaction
As of 7:46, MNKD stock jumped 9.51% to $4.49 with 715K shares traded hands.