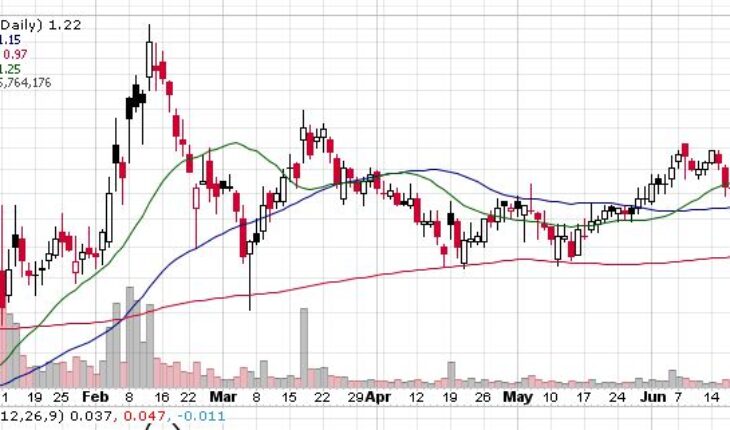
Tonix Pharmaceuticals Holding Corp (NASDAQ:TNXP) continues to move up in the pre-market session on Monday and is the leading gainer. The stock is up 114% over the past -month.
Major Trigger:
Tonix Pharmaceuticals Announces Program to Develop TNX-102 SL for the Treatment of Long COVID Syndrome, also Known as Post-Acute Sequelae of COVID-19 (PASC)
Key Highlights:
- announced it plans to develop TNX-102 SL (cyclobenzaprine HCl sublingual tablets) as a potential treatment for Long COVID Syndrome (Long COVID) which is now known officially as Post-Acute Sequelae of COVID-19 (PASC1). Tonix plans to meet with the U.S. Food and Drug Administration (FDA) in the third quarter of 2021 to seek agreement on the design of a potential Phase 2 pivotal study and the overall clinical development plan to qualify TNX-102 SL as an indicated treatment for Long COVID.
- Although most people recover from COVID-19 within weeks of the acute illness, a substantial portion develop a chronic syndrome called Long COVID, or PASC. These individuals experience a constellation of symptoms long past the time of recovery from acute COVID-19. Most Long COVID patients who have been studied appear to have cleared the SARS-CoV-2 virus from their systems.
- The symptoms of Long COVID can include fatigue, sleep disorders, pain, fevers, shortness of breath, cognitive impairment described as “brain fog”, gastrointestinal symptoms, anxiety, and depression. Long COVID can persist for months and can range in severity from mild to incapacitating. Several cohort studies have reported that persistence of symptoms following SARS-CoV-2 infection occurs in more than 30% of patients.2 While typically associated with moderate or severe COVID-19, Long COVID can occur after mild COVID-19 or even after asymptomatic SARS-CoV-2 infection.
Market Reaction
As of 8:10, TNXP stock gained by 3.30% to $1.26 So far more than 151K shares have exchanged hands.