Cocrystal Pharma Inc (NASDAQ:COCP) is trading with mild gains in the pre-market session after gaining 17% in the past month.
Major Trigger:
Cocrystal Pharma Completes IND-enabling Studies with CC-42344 for the Treatment of Seasonal and Pandemic Influenza A, Plans to initiate a Phase 1 Trial in the Third Quarter
Key Highlights:
- COCP announced the completion of IND-enabling studies with its potent, broad-spectrum PB2 inhibitor CC-42344 for the treatment of seasonal and pandemic influenza A and plans to initiate Phase 1 clinical development of CC-42344 in the third quarter of 2021.
- According to the World Health Organization (WHO) estimates, approximately 1 billion people are infected with seasonal influenza annually, resulting in 3 million to 5 million cases of severe illness and 250,000 to 500,000 deaths worldwide. Approved influenza therapies have limited efficacy due to drug resistance and viral mutation.
Key Quote:
“We are highly encouraged by the potential of CC-42344 to treat seasonal and pandemic influenza, both of which are major global health concerns,” said Sam Lee, Ph.D., Cocrystal’s President and interim co-CEO. “We recently completed a 14-day GLP toxicology study, which was the final pre-IND enabling step prior to advancing this potent inhibitor into a first-in-human study.
“There is a pressing need for new antivirals to treat influenza, as currently approved antiviral therapeutics are prone to viral resistance,” added Dr. Lee. “CC-42344 stops the first step of viral replication by binding to a highly conserved PB2 site of the influenza polymerase complex that is essential to replication. This uniquely positions CC-42344 to be an effective therapeutic against all significant A strains of the influenza virus, including avian pandemic strains as well as strains that are resistant to Tamiflu® (oseltamivir) and Xofluza® (baloxavir marboxil).”
Market Reaction
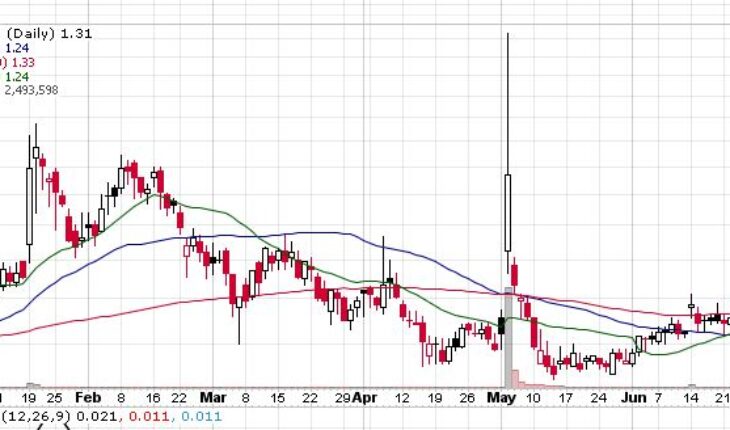
As of 8:41, COCP stock jumped 0.75% to $1.32 with 149k shares traded hands.