Tonix Pharma (NASDAQ:TNXP) gained 8% after the company revealed in a regulatory filing that it plans to construct a commercial-scale manufacturing plant for vaccine development and production, including its investigation COVID-19 vaccine, TNX-1800. The Montana facility, which will develop and manufacture TNX-1800, will be a private/public sector collaboration between the company and Ravalli County. The company said the collaboration would bring additional high-tech bioscience opportunities in Bitterroot Valley.
Tonix CEO Seth Lederman said that the COVID-19 pandemic exposed significant shortcomings in vaccine development and production capabilities domestically. Lederman said that the company is looking to pioneer vaccine development and manufacture in the US.
The company had announced plans to develop TNX-102 SL, a fibromyalgia candidate, as a possible l Long COVID syndrome treatment. Tonix also entered a licensing agreement with OyaGen for TNC-3500, a SARS-CoV-2 antiviral inhibitor currently under study for COVID 19 and other viral disorders treatment. In the coming months, TNX is a stock to keep an eye on.
Market Reaction:
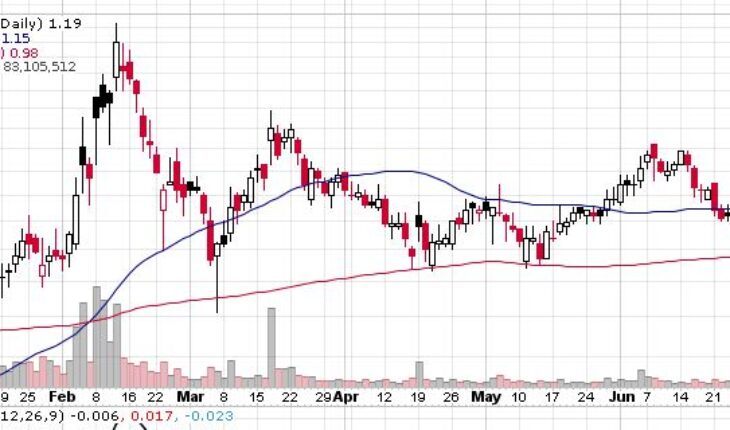
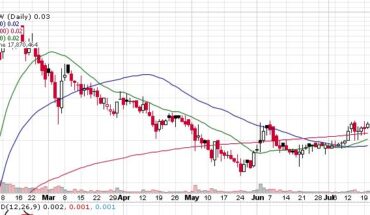
On Friday, TNXP stock jumped 8% to $1.19 with more than 83.10 million shares, compared to its average volume of 11.10 million shares. The stock has moved within a range of $1.1600 – 1.2900 after opening the trade at $1.21. Over the past 52-week, the stock has been trading within a range of $0.5100 – 2.4600.