Roche Holdings AG Basel ADR Common Stock (OTCMKTS: RHHBY) was up 0.79% on Friday and closed at $46.97. The Swiss multinational healthcare company announced that it received Emergency Use Authorization from the FDA for its treatment of intravenous Actemra/RoActemra® (tocilizumab) for COVID-19 treatment. This company has a market capitalization of $323.13 billion.
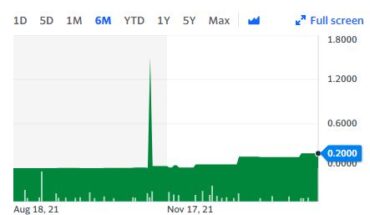
The day performance for RHHBY
After opening at $47.04, the stock exited at $46.97 with a share trading volume of 1.2K compared with its average share trading volume of 1.13M. It remained in the green zone throughout the day. Its day range was $46.88 – $47.09. RHHBY seems to be moving closer to its 52-week high of $48.38. Since May, Roche Holdings has gained 7.34%.
Receiving EUA for COVID-19 Treatment
Roche has received the EUA for its treatment for COVID-19 adults, and pediatric patients admitted in hospitals and receiving systemic corticosteroids. The treatment is authorized for the hospitalized patients in the category above who require supplemental oxygen, invasive or non-invasive mechanical ventilation, extracorporeal membrane oxygenation, among other supplies.
This EUA comes after results from controlled, randomized studies that evaluate Actemra/RoActemra for COVID-19 treatment in over 5,500 patients. The results have suggested improvement in patients receiving corticosteroids and were put on breathing support or supplemental oxygen.
It is worth noticing that despite the reduction in the total number of cases across the world, and the more extensive availability of vaccines, there is still hospitalization in severe forms for the disease. The drug, Actemra/RoActemra, has not yet been given a complete heads-up but is available only under an emergency access mechanism for certain kinds of patients.
Roche evaluated Actemra/RoActemra’s efficacy and safety in COVID-positive patients hospitalized under severe conditions in the clinical trial program.