Fortress Biotech (NASDAQ:FBIO) is trading flat in the pre-market session on Wednesday. The stock has jumped 35% over the past year.
Market Reaction
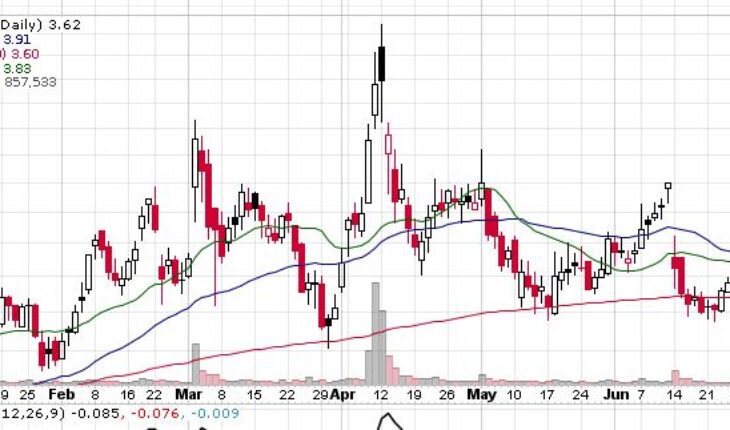
As of 7:45, FBIO is trading flay at $3.62 with 19k shares traded hands.
Major Trigger:
Journey Medical Corporation Enters into a Definitive Agreement with Dr. Reddy’s Laboratories Ltd. to Develop and Commercialize DFD-29 for the Treatment of Rosacea
Key Highlights:
- Journey Medical Corporation (“Journey Medical”), a partner company of Fortress Biotech, Inc. (NASDAQ: FBIO) (“Fortress”), today announced an agreement with Dr. Reddy’s Laboratories Ltd. (“Dr. Reddy’s”) for the collaborative development and commercialization of the DFD-29 program (Minocycline Modified Release Capsules 40 mg) for the treatment of rosacea. Journey Medical has acquired global commercialization rights including the U.S. and Europe, except that Dr. Reddy’s has retained certain rights to the program in select markets including Brazil, Russia, India and China.
- Through this collaboration, the parties will work together to complete the development of DFD-29. Dr. Reddy’s will provide development support including the monitoring of two Phase 3 clinical trials.
- A Phase 2 study in Germany was completed which assessed the efficacy, safety and tolerability of DFD-29 for the treatment of inflammatory lesions of rosacea over 16 weeks. DFD-29 demonstrated statistical significance to both placebo and active control, Oraycea® (German equivalent of U.S. marketed Oracea®), on both co-primary endpoints—proportion of subjects with Investigator’s Global Assessment treatment success (grade 0 or 1 with at least a two grade reduction from baseline at week 16) and total inflammatory lesion count reduction from baseline to week 16. Most notably, DFD-29 had approximately double the efficacy when compared against Oraycea® for both co-primary endpoints.