DiaMedica Therapeutics Inc. (NASDAQ:DMAC) dropped 34% despite the company announcing positive preliminary results from the second phase REDUX study of DM199 in treating chronic kidney disease.
The company had designed the study to evaluate DM199’s effects in their cohorts, including non-diabetic and hypertensive in cohort 1, IgA nephropathy in cohort two, and diabetic kidney disease in cohort 3. Results indicated that DM199 had clinically significant kidney function improvements in the first and second cohorts. In addition, DM199 reduces blood pressure in hypertensive participants.
Rick Pauls, DiaMedica CEO and President, said that the data offers clinical validation of the significant biologic activity of DM199 and sippers possibility of attaining clinical benefits better than or equal to the exogenous DM199 available in Asia.
He added that they are optimistic as they continue studying DM199 in hypertensive and IgA nephropathy African Americans with CKD with the pivotal phase 2/3 trial in acute ischemic stroke expected to commence this summer.
With these positive results and plans to advance the DM199 study, DMAC is a stock to watch in the coming months.
Market Reaction:
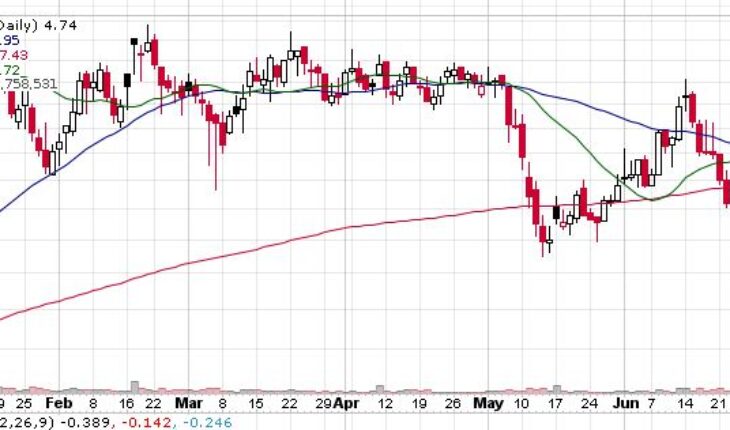
On Tuesday, DMAC stock plunged 34% to $4.74 with more than 4.75 million shares, compared to its average volume of 103k shares. The stock has moved within a range of $4.5900 – 5.2900 after opening the trade at $5.25. Over the past 52-week, the stock has been trading within a range of $3.7690 – 10.8800.