VistaGen Therapeutics (NASDAQ:VTGN) jumped 17.5% after releasing its fiscal 2021 results and offered a corporate update. However, it wasn’t the financial result that led to the biotech stock’s surge, considering it is yet to bring any commercial product to the market.
Instead, the company reported revenue of $1.1 million as part of the licensing agreement with EverInsight Therapeutics, combined last year with AffaMed Therapeutics. For the fiscal year ended March 31, 2021, the company reported a net loss of around $17.9 million.
The positive response from investors was attributed to VistaGen’s pipeline update. The company commenced the late-stage palisade-1 study recently evaluating its investigational drug PH94B in social anxiety disorder treatment. Study results will be available in mid-2022. The company is also planning to pick another later stage study, Palisade-2, this year.
In the coming months, investors will keep an eye on VTGN as the company moves to Palisade phase-3 clinical study. If the results will be promising, the company could file for regulatory approval in 2023.
Market Reaction:
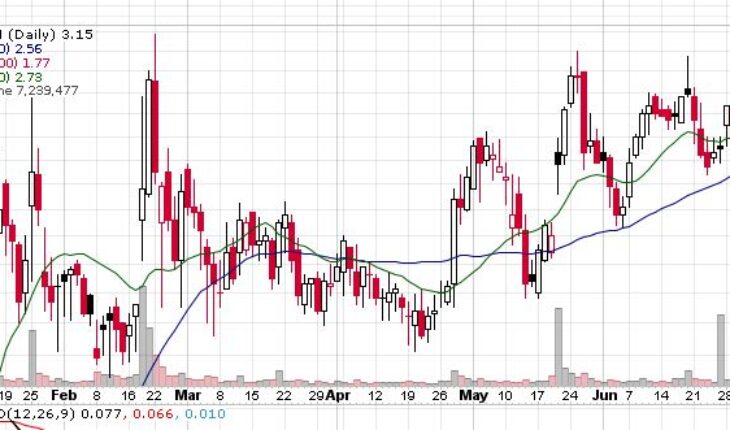
On Wednesday, VTGN stock soared 17.50% to $3.15 with more than 7.25 million shares, compared to its average volume of 2.66 million shares. The stock has moved within a range of $2.6200 – 3.1700 after opening the trade at $2.67. Over the past 52-week, the stock has been trading within a range of $0.4600 – 3.1800.