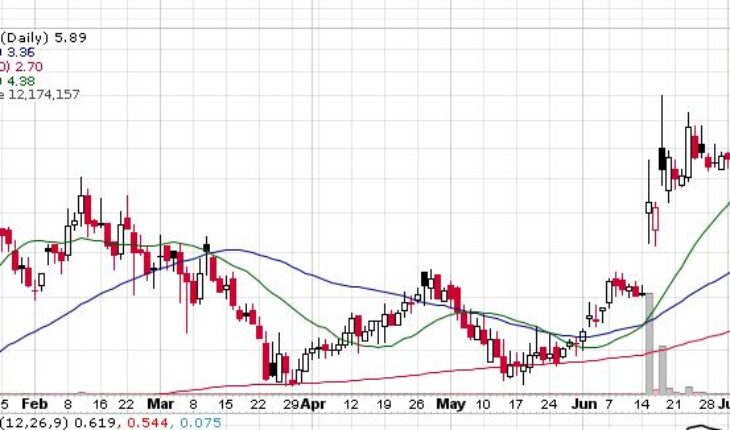
Shares of CLSD have soared 105% over the past month. Last month, Clearside Biomedical (NASDAQ:CLSD) came out with positive safety results on its Cohort 1 of the OASIS phase1/2a clinical trial of CLS-AX for treating neovascular age-related macular degeneration. The drug was givenusing Clearside’s SCS Microinjector in six patients with nAMD
The initial dose showed good toleration and did not have any adverse events and the firm said patients demonstrated no inflammation signs, no vasculitis or other issues. Average age of participants was 82 years old, with all patents having undergone anti-VEGF treatment through a set of injections prior to the trial.
Furthermore, all enrolled ones underwent diagnostic imaging at screening. The company said that mean central subfield thickness of macula was 231 µm with baseline best corrected visual acuity (BCVA) score at the start of the trial was 59.0.
The company has confirmed that after receiving CLS-AX, most patients demonstrated improvement in BCVA. Safety monitoring committee confirmed advancement to Cohort 2. Clearside Biomedical confirmed none of the patients in Cohort 1 needed extra treatment after one-month of giving the drug.
Patient screening for Cohort 2 with a dose of 0.1 mg CLS-AX will begin this month, with completion of the four-month trial period expected to commence by end of this year, the company reported.
Market Reaction:
As of 11:37, CLSD stock went up 10% at $5.92 with more than 12.03 million shares, compared to its average volume of 2.70 million shares. The stock has moved within a range of $5.35 – 7.29after opening the trade at $5.40. Over the past 52-week, the stock has been trading within a range of $1.25 – 7.29.