Last week, OncoSec Medical (NASDAQ:ONCS) has announced collaboration on phase 3 randomized clinical trial as well as supply arrangement with Merck. The aim of this trial (KEYNOTE-C87) is to assess survival of patients having late-stage metastatic melanoma. The patients are being treated with tavokinogenetelseplasmid (Tavo, OncoSec), DNA-plasmid interleukin (IL)-12, along with anti-PD-1 therapy pembrolizumab (Keytruda) vs standard of care.
This trial aims to facilitate approvel by FDA and serve as an important study for supporting complete licensure. Tavokinogenetelseplasmidgot Fast Track designationin February 2017 as an anti-cancer gene therapy for treating metastatic melanoma.
The clinical trial is intended to help accelerate approval by the FDA and serve as a pivotal study to support a full licensure. The agreement will entail Merck give pembrolizumab while OncoSec will give tavokinogenetelseplasmid, with each company bearing the costs and OncoSec also covering any third-party costs.
Eligible patients should have stage 3 or 4 metastatic melanoma and also be refractory to prior checkpoint therapy. KEYNOTE-C87 aims to enrol 400 patients from Canada, U.S, Australia and the EU.
Brian Leuthner, interim CEO of OncoSec, said that the agreement will ensure Tavo to patients as they may not have responded to the initial checkpoint inhibitor therapy. He added that the phase 3 collaboration is a milestone for OncoSecand joint progress with Merck having experienced team of immuno-oncology leaders will help patients with cancer.
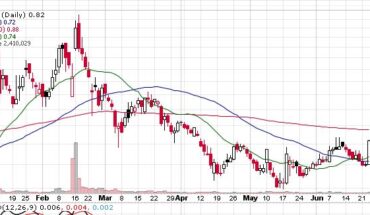
Market Reaction:
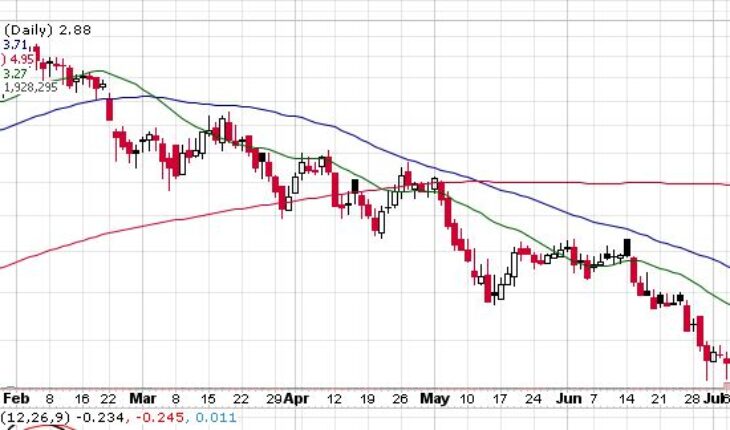
On Friday, ONCS stock slid 9.72% at $2.88 with more than 1.92 million shares, compared to its average volume of 1.60 million shares. The stock had moved within a range of $2.8100 – 3.1400 after opening the trade at $3.11. Over the past 52-week, the stock has been trading within a range of $2.0400 – 8.1600.