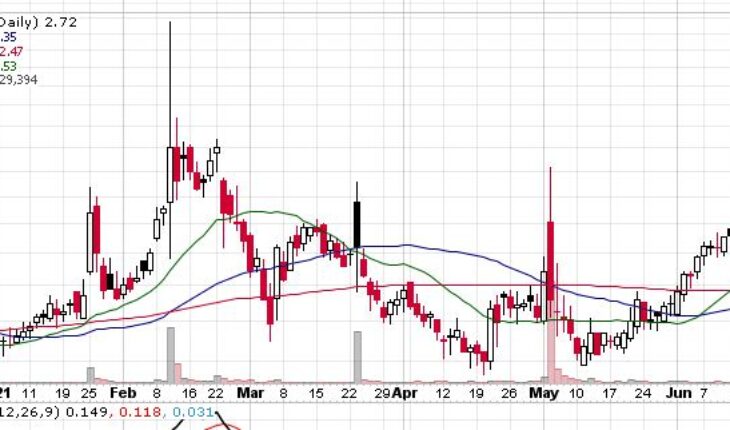
Plus Therapeutics Inc (NASDAQ:PSTV) is trading with mild gains in the pre-market session after gaining 28% in the past month.
Major Trigger:
Plus Therapeutics Announces DSMB Approval to Proceed into Eighth Cohort in ReSPECT™ Glioblastoma Trial
Key Highlights:
- Plus Therapeutics announced that the Data and Safety Monitoring Board recommended the Company proceed to the eighth cohort of the Phase 1 dose escalation ReSPECT™ trial, which is evaluating the Company’s lead investigational drug, Rhenium NanoLiposome (RNL™), in patients with recurrent glioblastoma (GBM).
- Twenty-one patients with recurrent GBM have been treated in the ReSPECT™ trial across seven cohorts to date. In these patients, RNL™ has had no dose-limiting toxicities observed with absorbed radiation doses of up to 740 Gray per tumor.
- The eighth cohort of the ReSPECT™ trial will implement a 40% increase in total radioactivity. The planned infused dose will be 31.2 millicuries in a volume of 12.3 milliliters (increased from 22.3 millicuries and 8.8 milliliters, respectively, used in cohort seven). The planned maximum flow rate will not change.
Key Quote:
“We are pleased with the favorable safety profile observed thus far with RNL™ in recurrent GBM,” said Marc Hedrick, M.D., President and Chief Executive Officer of Plus Therapeutics. “Moving to cohort eight will allow us to continue to push the envelope in terms of addressable tumor size and maximum radiation dose delivered.”
Market Reaction
As of 7:59, PSTV stock jumped 2.57% to $2.79 with 8K shares traded hands.