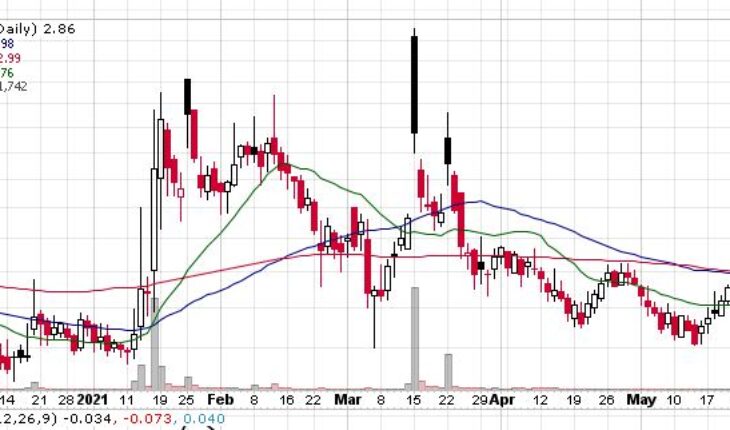
Acer Therapeutics Inc (NASDAQ: ACER) is moving a bit lower in pre-market trading.
ACER Release News
Acer Therapeutics Plans NDA Submission for ACER-001 in Q3 2021 Following Pre-NDA Meeting with FDA
- ACER announced the outcome of Acer’s pre-New Drug Application (NDA) meeting with the U.S. Food and Drug Administration (FDA) for ACER-001 for the treatment of Urea Cycle Disorders (UCDs). ACER-001 is a proprietary immediate release multi-particulate powder formulation of sodium phenylbutyrate (NaPB) with a taste-masked coating. ACER-001 is being developed in collaboration with Relief.
- The purpose of the pre-NDA meeting was to discuss the content of Acer’s planned NDA submission. Based on FDA feedback, the Companies believe the proposed data package will be sufficient to support an NDA submission under the Section 505(b)(2) regulatory pathway of ACER-001 for the treatment of patients with UCDs. Following NDA submission and FDA determination of acceptance for filing, the FDA will conduct a substantive review before deciding upon the action on the application.
“We are pleased with the outcome of our recent pre-NDA meeting with FDA, supporting our belief that the studies and data we intend to include in our planned NDA for ACER-001 should be sufficient for NDA submission,” said Chris Schelling, CEO and Founder of Acer. “We remain on track to complete the NDA submission in Q3 2021, provided that we obtain agreement with the FDA on our initial pediatric study plan (iPSP).”
Market Action
As of 9:10, APM is trading at $2.81 down 5 cents or 1.75%. So far more than 900 shares have exchanged hands.
Moving Averages
| +/- EMA(20) | 1.26 (+11.11%) |
| +/- SMA(50) | 1.36 (+2.94%) |
| +/- SMA(200) | 0.97 (+44.33%) |