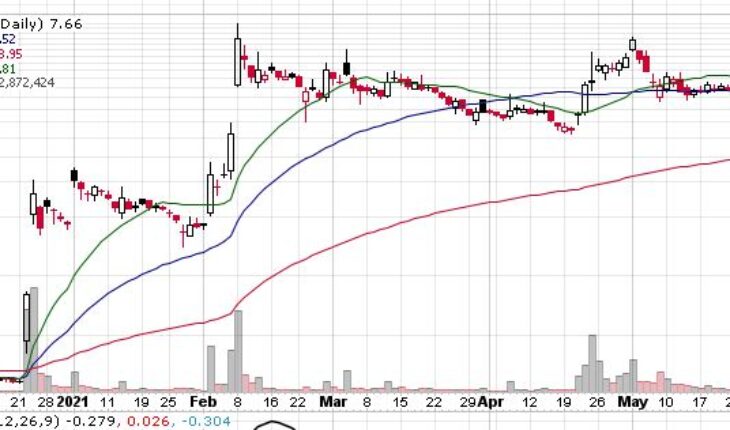
Ocugen Inc (NASDAQ:OCGN) is recovering from the recent sell off as the stock is up slightly up in the pre-market session.
Ocugen On Track to Submit Emergency Use Authorization Application to U.S. FDA for its COVID-19 Vaccine Candidate, COVAXIN™
- confirmed its plan to submit its Emergency Use Authorization (EUA) application for COVAXIN to the U.S. Food & Drug Administration (FDA) in June.
“Since we have been in discussions with the FDA since late last year, we do not believe that the FDA’s recently revised guidance regarding EUAs raises any concerns about our ability to submit the EUA for COVAXIN as planned, which is currently in process and which we expect to submit to the FDA in June.
We believe that the FDA’s new guidance confirms that Ocugen continues to meet all criteria for submission of an EUA. Once the EUA application has been submitted, Ocugen intends to commence pre-biologics license application (BLA) discussions with the FDA,” said Dr. Shankar Musunuri, Chairman of the Board, Chief Executive Officer, and Co-founder of Ocugen.
“FDA’s guidance refers specifically to vaccines based on the spike protein. COVAXIN is a unique yet traditional vaccine using an inactivated version of the whole virus with a novel adjuvant that provides a broadly protective immune response beyond the spike protein, offering potential effectiveness against multiple existing and emerging variants and reducing the possibility of mutant virus escape,” said Dr. Bruce Forrest, Acting Chief Medical Officer and member of the vaccine scientific advisory board of Ocugen.
Market Action
OCGN stock has moved up by 17 cents or 2.22% to $7.83 in the pre-market session with more than 1.59 million share traded hands.