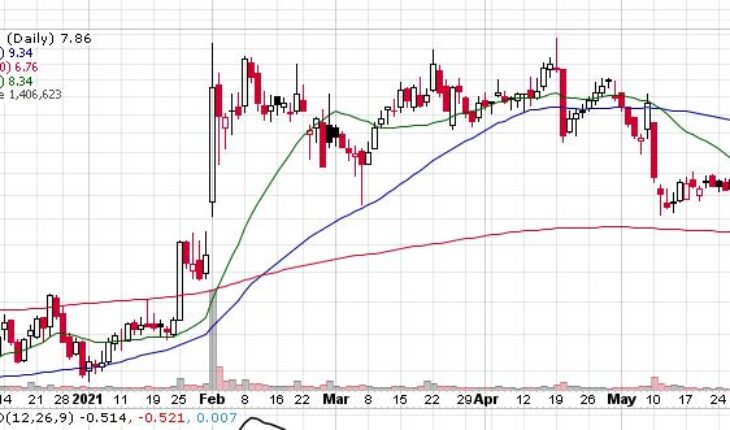
Dynavax Technologies Corporation (NASDAQ:DVAX) is moving up with a bit in the pre-market session following the news.
Dynavax Announces Agreement with Bavarian Nordic for Commercialization of HEPLISAV B®, a Two Dose Adult Hepatitis B Adjuvanted Vaccine, in Germany
- announced it has entered into a commercialization agreement with Bavarian Nordic for the marketing and distribution of HEPLISAV B [Hepatitis B Vaccine (Recombinant), Adjuvanted] in Germany with an expected launch in the fourth quarter of 2021.
- In February 2021, the European Commission (EC) granted marketing authorization for HEPLISAV B for the active immunization against hepatitis B virus infection (HBV) caused by all known subtypes of hepatitis B virus in adults 18 years of age and older. HEPLISAV B is the only U.S. Food and Drug Administration (FDA) and EC approved hepatitis B vaccine for adults with a two-dose regimen that is completed in one month.
- Hepatitis B is a highly infectious and potentially deadly virus with increasing infection rates, and over 250 million people infected worldwide. Hepatitis B can be prevented with effective vaccination. HEPLISAV B, with a two-dose regimen that takes only one month to complete and a statistically significantly higher seroprotection rate in head-to-head clinical trials, provides a unique opportunity to address known challenges with compliance, while delivering higher levels of protection compared to the three-dose regimen of the comparator vaccine.
Paul Chaplin, President and CEO of Bavarian Nordic, commented: “We are pleased to expand our commercial footprint in the largest EU market by adding a complementary product to our marketing and distribution and we look forward to assisting Dynavax in a successful market entry in Europe later this year.”
Market Action
As of 8:46, DVAX stock went up by $0.16 or 2.04% to $8.02 on heavy volume of 37K shares.