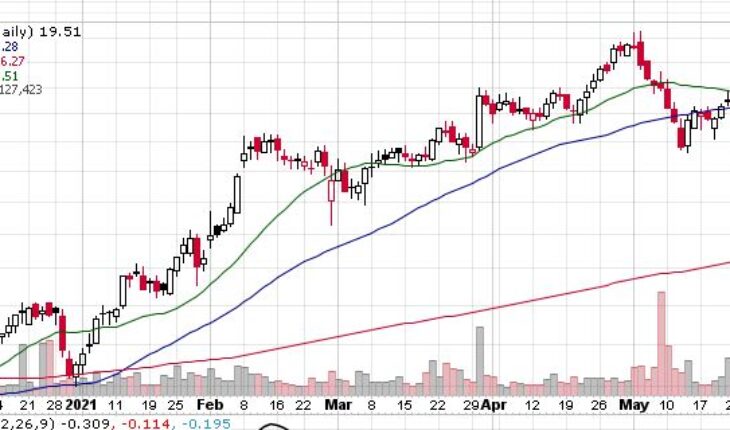
Lantheus Holdings (NASDAQ:LNTH) is moving up with a double-digit rally in the pre-market session after the news.
Lantheus Receives U.S. FDA Approval of PYLARIFY® (piflufolastat F 18) Injection, the First and Only Commercially Available PSMA PET Imaging Agent for Prostate Cancer
- LNTH announced today that the U.S. Food and Drug Administration (FDA) has approved PYLARIFY, an F 18-labeled prostate-specific membrane antigen (PSMA) targeted positron emission tomography (PET) imaging agent to identify suspected metastasis or recurrence of prostate cancer.
- PYLARIFY is the first and only commercially available approved PSMA PET imaging agent for prostate cancer.
- The product will be immediately available in parts of the mid-Atlantic and southern regions and availability is expected to rapidly expand over the next six months with broad availability across the U.S. anticipated by year end.
“The FDA approval of PYLARIFY is a significant milestone for Lantheus and the prostate cancer community in the United States. We believe PYLARIFY represents a paradigm shift in the identification and management of patients with suspected metastasis or recurrent prostate cancer, providing more accurate and earlier detection of disease than conventional imaging so that doctors, along with patients and their families, can make more informed treatment decisions,” said Mary Anne Heino, President and Chief Executive Officer of Lantheus.
Market Action
As of 8:46, LNTH stock decreased by $2.98 or 15.27% to $22.49 on heavy volume of 333K shares.