Tonix Pharmaceuticals Holding Corp (NASDAQ:TNXP) stock opens slightly lower after gaining momentum recently.
Market Action
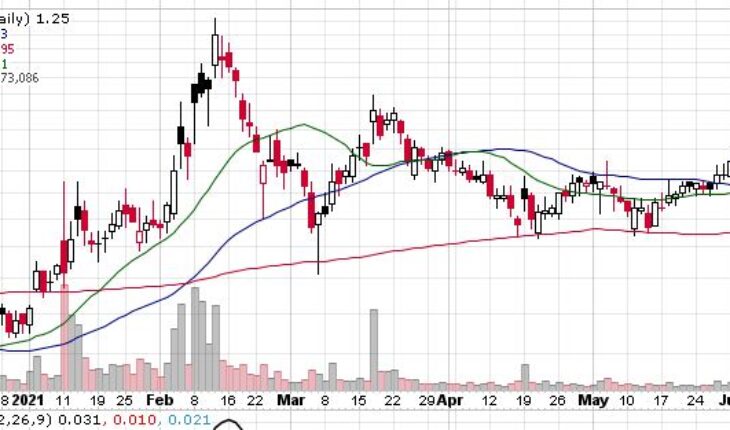
As of 10:00, TNXP stock fell 0.80% to $1.23 with more than 2.52 million shares, compared to its average volume of 14.20 million shares. The stock has moved within a range of $1.2000 – 1.2600 after opening the trade at $1.24.
Tonix Pharma (TNXP) Presents Positive Results from Phase 3 RELIEF Study of TNX-102 SL for the Management of Fibromyalgia at ASCP
- announced the poster presentation of positive results from its Phase 3 clinical study, RELIEF, of TNX-102 SL for the management of fibromyalgia. A copy of the poster will be made available under the IR Events tab of the Investors section of the Tonix website at www.tonixpharma.com.
- The poster, titled, “Efficacy and Safety of TNX-102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia in the RELIEF Study: Positive Results of a Phase 3 Randomized, Double-Blind, Placebo-Controlled Multicenter Trial” shows that TNX-102 SL met its pre-specified primary endpoint in the Phase 3 RELIEF trial, significantly reducing daily pain compared to placebo (p=0.01) and was associated with a higher rate than placebo of ≥30% pain responders in participants with fibromyalgia (p=0.006). TNX-102 SL at 5.6 mg also showed activity in key secondary endpoints measuring improvements in sleep quality, mitigation of fatigue, and fibromyalgia-specific functional recovery. In addition, TNX-102 SL was well tolerated and was not associated with side-effects seen with other approved oral fibromyalgia treatments, including weight gain, insomnia, nausea or sexual dysfunction.
“We believe the results of the Phase 3 RELIEF trial validate the mechanism that improved sleep quality can lead to syndromal effects on fibromyalgia, improving not only pain but also sleep and fatigue. The sublingual formulation of TNX-102 SL for transmucosal absorption showed promise at the 2.8 mg dose in prior fibromyalgia studies, but we believe RELIEF provides evidence that 5.6 mg is the right dose for this patient population,” said Seth Lederman, M.D., President and Chief Executive Officer. “We expect interim analysis results for the confirmatory Phase 3 study, RALLY, in the third quarter of this year, followed by topline data in the first quarter of next year.”