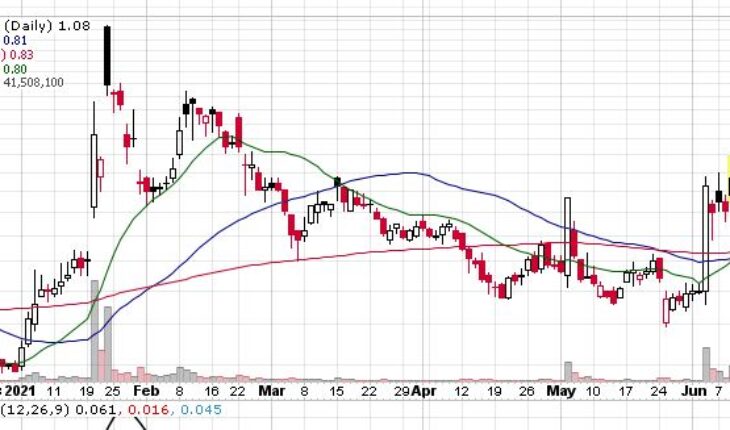
The Adamis Pharmaceuticals (NASDAQ:ADMP) stock has made a big move this morning and went up by 18% after the United States Food and Drug Administration accepted its resubmitted New Drug Application for the product Zimhi.
The product is meant for treating those suffering from a case of opioid overdose. In addition to that, that FDA has also provided Adamis with the PDUFA action date of November 12, 2021.
After the filing review had been completed by the FDA, it determined that the new application was complete enough to deserve a review. The resubmission had been made by Adamis in May, 2021. It is a major development for Adamis and one that has been recognised by the market as well. Dr. Dennis J. Carlo, who is the CEO and President of the company, stated that the company is eager to collaborate with the FDA throughout the course of the review process.
Technical Indicators
| Symbol | Value | Result | Symbol | Value | Result |
| ADX | 23.7932 | Neutral | ATR14 | 0.1247 | |
| CCI20 | 162.1182 | Buy | Chaikin Money Flow | 0.3286 | Buy |
| MACD | 0.0415 | Buy | Money Flow Index | 69.9713 | Buy |
| ROC | 31.9916 | Buy | RSI | 60.6040 | Sell |
| STOCH (14,3) | 68.5950 | Buy | STOCH RSI | 0.6264 | Neutral |
| UO | 59.6333 | Buy | Williams %R | -31.4050 | Neutral |