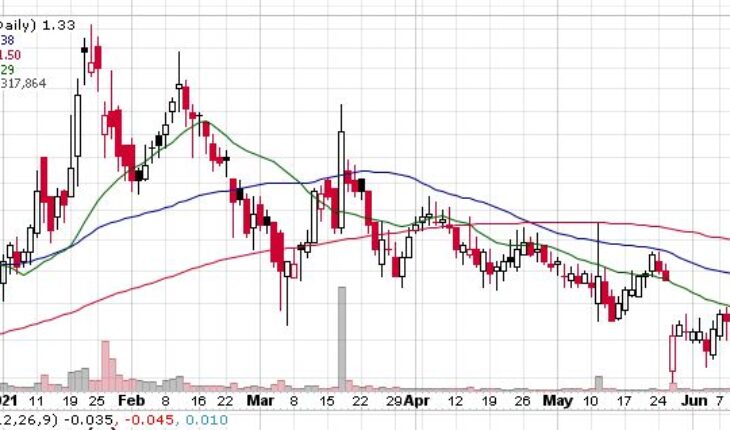
Lipocine Inc (NASDAQ:LPCN) is trading up in the pre-market session on Thursday. The stock has been witnessing solid rally over the past week with a jump 10%.
Market Reaction
As of 8:47, LPCN is up by 8% to $1.45 with 340K shares traded hands.
Major Trigger:
FDA Clears LPCN 1154 IND Application for a Phase 2 Postpartum Depression Study
Key Highlights:
today announced that the U.S. Food and Drug Administration (“FDA”) has cleared the Company’s Investigational New Drug Application (“IND”) to initiate a Phase 2 study to evaluate the therapeutic potential of LPCN 1154, an oral neuro-steroid product candidate, for the treatment of postpartum depression (“PPD”) in adults.
A pharmacokinetic (“PK”) study to assess dose proportionality is planned to start in July 2021 with top-line results expected in the third quarter of 2021.
Following the PK study, a proof-of-concept study to evaluate the safety, tolerability, and efficacy of LPCN 1154 in adult female subjects diagnosed with PPD is expected to occur with the first subject dosed in the fourth quarter of 2021.
PPD, a major depressive disorder that is under diagnosed in US, impacts approximately 1 in 7 women after giving birth. PPD can lead to devastating consequences for a woman, her newborn and her family. Currently, there is no oral therapy approved for the treatment of PPD. The active moiety in LPCN 1154 is an endogenous positive allosteric modulator of γ-aminobutyric acid (“GABAA”) receptor.
LPCN 1154 is expected to be an “at home” treatment with easier treatment access than the current standard of care invasive option that requires hospitalization with significant limitations. Moreover, LPCN 1154 is expected to provide the required level of privacy for a mother, avoiding bonding/breast feeding interruptions due to the required hospitalizations for the current option.