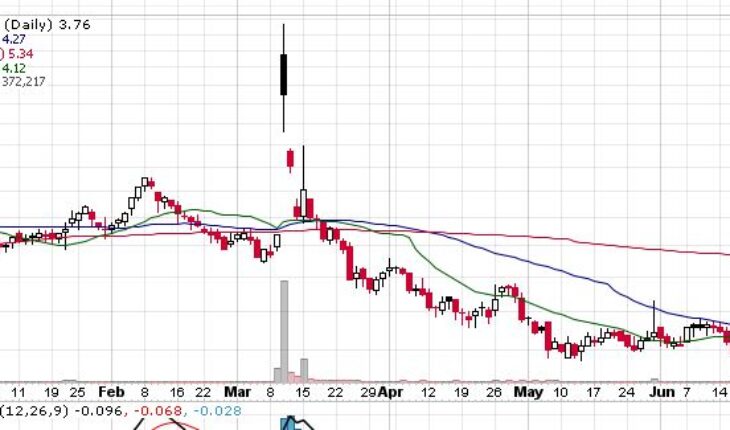
MediciNova, Inc. (NASDAQ:MNOV) is one of the biggest gainers in the pre-market session on Monday after announcing the announces positive results from phase 2 trial of MN-166.
Major Trigger:
MediciNova Announces Positive Results from Phase 2 Trial of MN-166 (ibudilast) in Alcohol Use Disorder Published in Nature’s Translational Psychiatry Journal
Key Highlights:
- MNOV announced that positive results from a Phase 2 trial of MN-166 (ibudilast) in alcohol use disorder (AUD) were published in Nature’s Translational Psychiatry.
- The clinical trial was a collaborative effort between MediciNova and Dr. Lara Ray, Professor, Department of Psychology and Department of Psychiatry and Biobehavioral Sciences, Brain Research Institute at the University of California at Los Angeles (UCLA) and was funded by the Center for Study of Opioid Receptors and Drugs of Abuse (CSORDA; National Institute on Drug Abuse Grant P50-DA005010). This study was a randomized, double-blind, placebo-controlled Phase 2 trial to evaluate the effect of 14 days of ibudilast treatment on mood, heavy drinking, and neural reward signals in individuals with AUD. A total of 52 AUD patients were enrolled in this trial.
- The publication, entitled “Ibudilast, a neuroimmune modulator, reduces heavy drinking and alcohol cue-elicited neural activation: a randomized trial” was authored by Dr. Lara Ray and colleagues at UCLA.
Key Quote:
Kazuko Matsuda, MD, PhD, MPH, Chief Medical Officer of MediciNova, Inc., commented, “We are very pleased that the results from this Phase 2 trial in alcohol use disorder have been published. We are excited that MN-166 reduced the odds of heavy drinking by 45% after only 14 days of treatment. Our first clinical trial demonstrated that ibudilast significantly reduced basal, daily alcohol craving in AUD patients. We are thrilled that MN-166 has demonstrated great potential to reduce the increasing problem of alcohol use disorder.”
Market Reaction
As of 6:57, MNOV is trading at $42, up 42%. So far more than 10K shares have exchanged hands.