MediWound Ltd. (NASDAQ:MDWD) plunged 28% after the FDA issued the company a Complete Response Letter about its Biologics License Application for NexoBird’s approval in eschar removal in patients with full-thickness or deep partial-thickness thermal burns. The FDA notified the company that it had concluded the BLA review as amended and determined that it cannot approve the application in its current form. In addition, the regulatory agency identified issues in the Chemistry, Manufacturing, and Controls (CMC) section of the application and requested more CMC information.
The FDA acknowledged that it had received several SMC amendments in response to CMC information requested but didn’t review the amendments for this decision. According to the agency, an inspection of the manufacturing facilities of NexoBird in Taiwan and Israel is necessary before it can approve the BLA.However, the COVID-19 pandemic-travel-related restrictions hindered the inspection.
MediWound CEO Sharon Malka said that the decision is disappointing, but they are confident in their solid clinical data and depth of the development program. In the coming months, MDWD is a stock to watch.
Market Reaction:
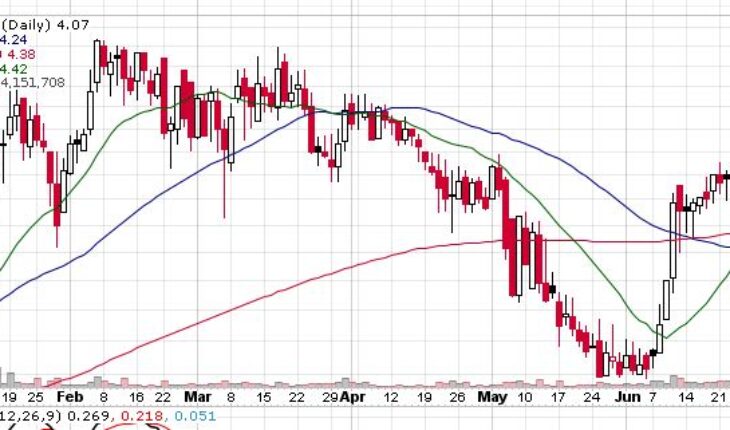
On Tuesday, MDWD stock slumped 28% to $4.07 with more than 4.14 million shares, compared to its average volume of 211k shares. The stock has moved within a range of 3.7100 – 4.2700 after opening the trade at $3.75. Over the past 52-week, the stock has been trading within a range of $2.9000 – 6.2200.