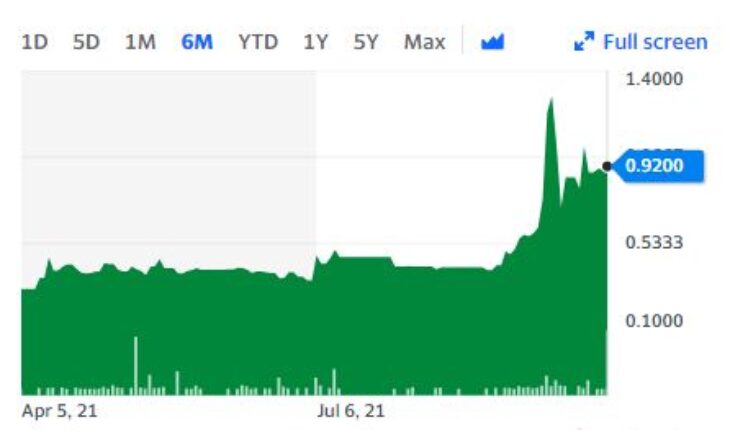
Innocan Pharma Corporation (OTCMKTS:INNPF) on October 4, 2021, announced that a fresh patent application has been done for its CBD Delivery System Technology along with CLX (CBD Loaded Exosomes) and existing LTP (CBD Loaded Liposomes).
The new patent application reveals a unique delivery system enabling the controlled release of CBD into the bloodstream with improved pharmacokinetic performance. This patent is an important milestone in research done jointly with Hebrew University demonstrating the potential of the firm’s capability of delivering cannabinoids to the bloodstream in accurate administration.
Controlled and prolonged release of Cannabidiol from its unique Delivery system demonstrated continuous clinically relevant concentrations of CBD for a prolonged period. The firm believes this is a good part of expected CBD exposure in humans.
The firm is of the view that CBD in the blood is better than orally administered CBD in two aspects. Firstly, it allows single administration instead of daily, and secondly, it overcomes the low (10-20%) oral bioavailability of CBD.
Prof.ChezyBarenholz of The Hebrew University of Jerusalem said that the filing of the PCT is an extra step towards its R&D scientific achievement. Barenholz added that the firm is proud on enabling cutting-edge solutions which can serve as a foundation to CBD Pharma in the future.
Iris Bincovich, CEO of Innocan, said that the publication of the PCT patent application shows the uniqueness of the approach as well as the advantage.