CytoDyn Inc (OTCMKTS:CYDY) filed an 8K after market hours regarding an exclusive distribution agreement.
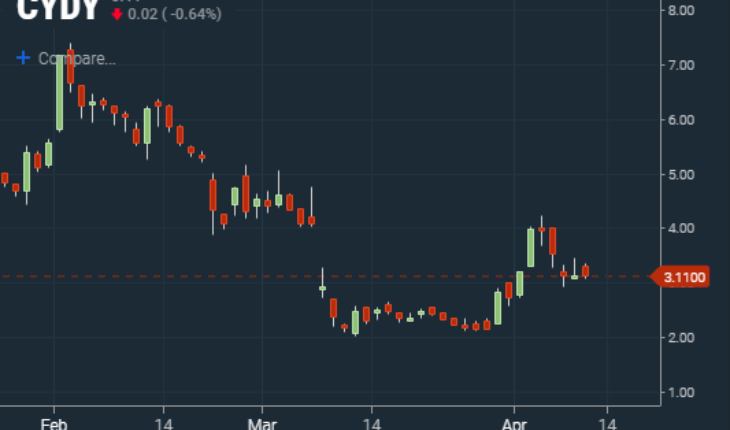
CytoDyn Inc (OTCMKTS:CYDY) today traded slightly lower today down .02 to 3.11 Volume was a very light 2.1 million shares well below its 30 day average of 5.2 million shares a day. Potential pivot points to keep an eye on in CYDY may be 3.30, 3.47, and 3.77 above and 3, 2.88 and 2.59 below.
Highlights from the 8k filings
On April 6, 2021, CytoDyn Inc. (the “Company”) entered into an Exclusive Supply and Distribution Agreement (the “Agreement”) with Biomm S.A., a Brazilian pharmaceutical company engaged in the business of manufacturing and distributing pharmaceutical products in Brazil (“Biomm”), pursuant to which Biomm would hold the exclusive right to distribute and sell the Company’s product, Vyrologix™ (leronlimab), in Brazil, once regulatory approval has been received. The Agreement provides for the sale of Vyrologix upon approval by the Brazilian National Health Surveillance Agency or Agência Nacional de Vigilância Sanitária (“ANVISA”).
Under the Agreement, the Company has appointed Biomm as the exclusive distributor for Vyrologix in Brazil, agreed to supply Vyrologix to Biomm, and agreed not to supply Vyrologix or the rights to import, distribute, resell or market Vyrologix to any other public or private entity in Brazil without Biomm´s consent and participation. Biomm is responsible for submitting an application for Authorization for Emergency Use for COVID-19 treatment in accordance with the laws and regulations in Brazil following regulatory clearance. Biomm will, at its cost, with the assistance of the Company, prepare the transfer, translation and interpretation of the relevant data and materials submitted by the Company to the U.S. Federal Drug Administration to the extent necessary to complete the relevant filings with ANVISA and all applicable local regulatory agencies, and translate the proposed label and summaries of the clinical information for filing with the local healthcare regulatory authorities and all other applicable regulatory authorities, and take such other actions, at its own cost, as are necessary to obtain and maintain all governmental approvals, authorizations, licenses, permits, registrations and consents that are, or may in the future be, required for the parties to perform under the Agreement.
The term of the Agreement will remain in effect until formal regulatory approval for the sale of Vyrologix in Brazil has been received. For application of formal regulatory approval of Biomm as the marketing authorization holder in Brazil, the Company and Biomm agree to amend the Agreement to describe the specific regulatory and commercial terms of approval. Either Party may terminate the Agreement (i) for cause, if the other party has materially breached any of its obligations and has not cured such breach after being given the reasonable opportunity to do so, (ii) upon the bankruptcy or insolvency of the other party, (iii) upon continuation of a force majeure event that prevents performance of the other party for more than 120 days, (iv) in the event that ANVISA makes a final, non-appealable decision to not approve Vyrologix for emergency use or withdraws approval of its emergency use authorization, or (v) if the other party’s license to conduct its business expires, is not renewed, or is revoked or suspended, or such party becomes legally disqualified for any reason from importing, exporting, distributing, promoting or selling Vyrologix in Brazil, or otherwise from performing its obligations under this Agreement.
The above description of the Agreement does not purport to be complete and is qualified in its entirety by the full text of the Agreement, which the Company intends to file as an exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended May 31, 2021.