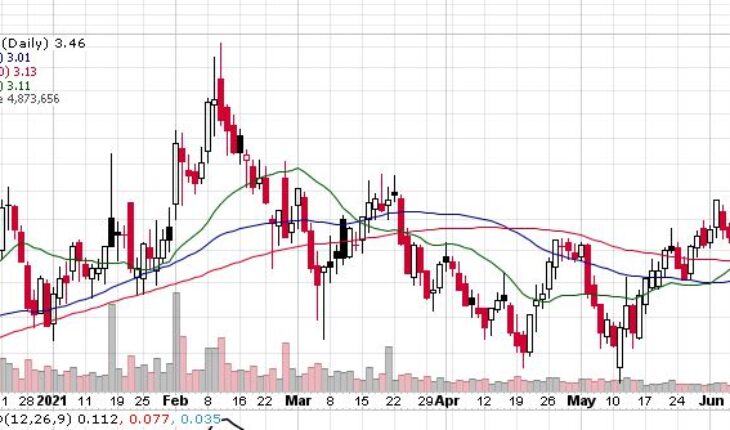
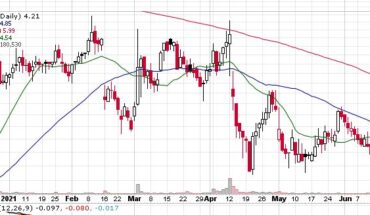
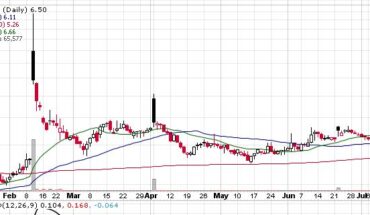
VBI Vaccines Inc (NASDAQ:VBIV) continues to gain momentum as the stock is heading for another green day after yesterday’s jump of 5.50%.
Major Trigger:
VBI Vaccines Granted FDA Fast Track Designation for VBI-1901 for the Treatment of Recurrent GBM
Key Highlights:
- Today announced that the U.S. Food and Drug Administration (FDA) granted Fast Track Designation for VBI-1901, VBI’s cancer vaccine immunotherapeutic candidate for the treatment of recurrent glioblastoma (GBM) patients with first tumor recurrence. Fast Track Designation facilitates the development and expedites the review of new therapies to treat serious conditions and fill an unmet medical need.
- FDA’s Fast Track Designation for VBI-1901 underscores the significant and urgent unmet medical need for new therapies for recurrent glioblastoma (GBM) patients
- Designation granted based on data from the Phase 1/2a study of VBI-1901 in recurrent GBM patients
- Most recent tumor response data and overall survival (OS) data from Phase 1/2a study were presented in a poster at American Society of Clinical Oncology (ASCO) Annual Meeting, June 4-8, 2021
Key Quote
“This Fast Track Designation provides additional medical validation and is a meaningful milestone for our development of VBI-1901 as we work to provide clinical benefit for patients who have few treatment options available today,” said Francisco Diaz-Mitoma, M.D., Ph.D., VBI’s Chief Medical Officer. “Building on the encouraging data seen to-date – including updated tumor responses and improvement in overall survival compared to historical controls as presented at ASCO – we look forward to working closely with the FDA as we progress this cancer vaccine immunotherapeutic candidate with the hope of improving outcomes for adults with recurrent GBM.”
Market Reaction
As of 8:25, VBIV stock is higher by 6.36% at $3.68. The stock has traded 519K shares so far in the pre-market session.