After it provided a joint statement with its collaboration partner Acer Therapeutics Inc yesterday, the Relief Therapeutics Holding SA (OTCMKTS:RLFTF) stock came into focus among investors and ended up with gains of as much as 12% as a consequence.
Market Action
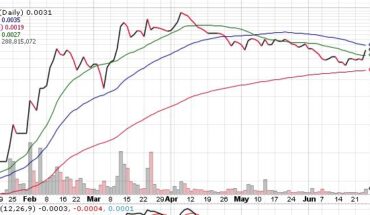
On Tuesday, RLFTF stock soared 11.67% to $0.0335 with more than 5.09 million shares, compared to volume of 1.04 million shares. The stock moved within a range of $0.0284 – 0.0348 after opening trade at $0.0310.
Acer Therapeutics and Relief Therapeutics Announce U.S. FDA Approval of OLPRUVA™ for Patients with Urea Cycle Disorders
It may be a good idea for investors to actually take a look at the announcement that was made. The statement noted that the product OLPRUVA had been approved by the United States Food and Drugs Administration for oral suspension in the country for treating some patients suffering from urea ulcer disorders. It goes without saying that the development was a major one for Relief Therapeutics and it is going to be interesting to see if the stock stays in focus among investors over the course of the coming days.
The reaction from investors was also proof of the fact that there was significant excitement about the news. In addition to that, the approval also had an added impact. It would also help in the triggering of a loan that would see Acer get as much as $42.5 million term loan that had been announced back in March this year. It may be a good time to add Relief in your watch lists.
Key Quote
“The FDA’s approval of OLPRUVA™, an innovative formulation of sodium phenylbutyrate packaged for the first time in single-dose envelopes, marks the culmination of our ongoing dedication to develop new and differentiated treatment options for those affected by rare diseases,” said Chris Schelling, chief executive officer and founder of Acer. “Patients who are living with UCDs now have an alternative treatment option with OLPRUVA™, to address some of the challenges they may have with existing therapy. We are pleased to be able to provide a new, approved treatment choice for those living with this challenging disease.”